The Mass Spectrometric Ortho Effect for Distinguishing the Coeluting Isomers of Polychlorinated Biphenyls and the Coeluting Isomers of Polybrominated Biphenyls: Qualitative and Quantitative Aspects
- PMID: 39124889
- PMCID: PMC11314496
- DOI: 10.3390/molecules29153484
The Mass Spectrometric Ortho Effect for Distinguishing the Coeluting Isomers of Polychlorinated Biphenyls and the Coeluting Isomers of Polybrominated Biphenyls: Qualitative and Quantitative Aspects
Abstract
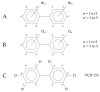
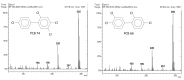
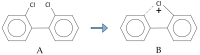
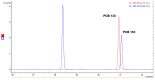
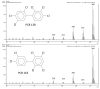
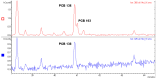
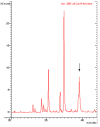
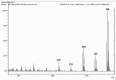
Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) are persistent organic pollutants still widespread in the environment and in the food chain. Both groups of these synthetic xenobiotics consist of 209 possible congeners depending on the number and position of halogens. PCBs with the same number of chlorine atoms and PBBs with the same number of bromine atoms are isomers: ten different degrees of halogenation are allowed, which results in a lot of existing isomers for both groups. The isomers have perfect correspondence in the number and type of atoms with differences only in positioning, so their mass spectra are expected to be identical with a consequent significant analytical problem in the event of coelution of the chromatographic peaks. This is not always the case, since the mass spectrometric ortho effect is capable of effectively discriminating many coeluting PCB or PBB isomers, although not all possible ones. The present paper investigates, for the first time, the reliability of qualitative and quantitative analysis by using the ortho effect: this was conducted through targeted experimental measurements on real samples of food by using different detectors. In this context, it is shown how to recognize the presence of a PCB that does not have the ortho effect when coeluting with an isomer that has. This is an important aspect that has never been studied until now. The ortho effect is extremely simple to operate once the ordinary GC-MS runs have been performed: the analyst only needs to recheck the mass spectrum for measuring the intensity of the first dehalogenation ion. The topic is of practical relevance since two different isomers can have different health hazards, and the presence of a very toxic isomer could be masked by a less toxic one. The same mass spectrometric ortho effect also deals with PXBs (i.e., mixed poly-brominated/chlorinated biphenyls), which are emerging contaminants.
Keywords: analytical tool; coelution of isomers; emerging contaminants; mass spectrometry; metrology; mixed poly-brominated/chlorinated biphenyls (PXBs); ortho effect; polybrominated biphenyls (PBBs); polychlorinated biphenyls (PCBs).
Conflict of interest statement
The author declares no conflicts of interest.
Figures

Similar articles
-
Use of the 'ortho effect' for chlorinated biphenyl and brominated biphenyl isomer identification.Biomed Environ Mass Spectrom. 1987 Oct;14(10):579-82. doi: 10.1002/bms.1200141008. Biomed Environ Mass Spectrom. 1987. PMID: 2827819
-
Mixed poly-brominated/chlorinated biphenyls (PXBs): widespread food and environmental contaminants.Environ Int. 2012 Sep;44:118-27. doi: 10.1016/j.envint.2012.03.006. Epub 2012 Apr 6. Environ Int. 2012. PMID: 22483842 Review.
-
Fish contamination by polychlorobiphenyls: the mass spectrometric ortho effect in a new and easy gas chromatography-mass spectrometry method for the analysis of the seven indicators. The case of Bluefin Tuna.J Chromatogr A. 2015 Jan 2;1375:110-22. doi: 10.1016/j.chroma.2014.11.016. Epub 2014 Nov 13. J Chromatogr A. 2015. PMID: 25498559
-
pK(a) values of the monohydroxylated polychlorinated biphenyls (OH-PCBs), polybrominated biphenyls (OH-PBBs), polychlorinated diphenyl ethers (OH-PCDEs), and polybrominated diphenyl ethers (OH-PBDEs).J Environ Sci Health A Tox Hazard Subst Environ Eng. 2010 Sep;45(11):1322-46. doi: 10.1080/10934529.2010.500885. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2010. PMID: 20658412
-
Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): biochemistry, toxicology, and mechanism of action.Crit Rev Toxicol. 1984;13(4):319-95. doi: 10.3109/10408448409023762. Crit Rev Toxicol. 1984. PMID: 6091997 Review.
References
-
- Polychlorinated Biphenyls and Polybrominated Biphenyls. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [(accessed on 21 June 2024)];2015 Volume 107 Available online: https://publications.iarc.fr/131. - PMC - PubMed
-
- Jacobson M.H., Darrow L.A., Boyd Barr D., Howards P.P., Lyles R.H., Terrell M.L., Smith A.K., Conneely K.N., Marder M.E., Marcus M. Serum polybrominated biphenyls (PBBs) and polychlorinated biphenyls (PCBs) and thyroid function among Michigan adults several decades after the 1973–1974 PBB contamination of livestock feed. Environ. Health Persp. 2017;125:097020. doi: 10.1289/EHP1302. - DOI - PMC - PubMed
-
- Stockholm Convention, Alternatives to POPs, Chemicals Listed in Annex A, Hexabromobiphenyl. [(accessed on 26 June 2024)]. Available online: https://chm.pops.int/Implementation/Alternatives/AlternativestoPOPs/Chem....
LinkOut - more resources
Full Text Sources
Miscellaneous

