Structural Unfolding of G-Quadruplexes: From Small Molecules to Antisense Strategies
- PMID: 39124893
- PMCID: PMC11314335
- DOI: 10.3390/molecules29153488
Structural Unfolding of G-Quadruplexes: From Small Molecules to Antisense Strategies
Abstract
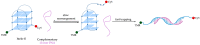
G-quadruplexes (G4s) are non-canonical nucleic acid secondary structures that have gathered significant interest in medicinal chemistry over the past two decades due to their unique structural features and potential roles in a variety of biological processes and disorders. Traditionally, research efforts have focused on stabilizing G4s, while in recent years, the attention has progressively shifted to G4 destabilization, unveiling new therapeutic perspectives. This review provides an in-depth overview of recent advances in the development of small molecules, starting with the controversial role of TMPyP4. Moreover, we described effective metal complexes in addition to G4-disrupting small molecules as well as good G4 stabilizing ligands that can destabilize G4s in response to external stimuli. Finally, we presented antisense strategies as a promising approach for destabilizing G4s, with a particular focus on 2'-OMe antisense oligonucleotide, peptide nucleic acid, and locked nucleic acid. Overall, this review emphasizes the importance of understanding G4 dynamics as well as ongoing efforts to develop selective G4-unfolding strategies that can modulate their biological function and therapeutic potential.
Keywords: G-clamp; G-quadruplex; antisense strategy; disrupting small molecules; locked nucleic acid; new therapeutic strategies.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures








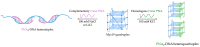



References
-
- Zuffo M., Pirota V., Doria F. Photochemistry: Volume 46. Volume 46. The Royal Society of Chemistry; London, UK: 2019. Photoresponsive molecular devices targeting nucleic acid secondary structures; pp. 281–318. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

