Substituent Effects in the Photophysical and Electrochemical Properties of Meso-Tetraphenylporphyrin Derivatives
- PMID: 39125093
- PMCID: PMC11314014
- DOI: 10.3390/molecules29153689
Substituent Effects in the Photophysical and Electrochemical Properties of Meso-Tetraphenylporphyrin Derivatives
Abstract
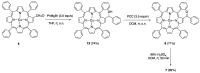
Porphyrins were identified some years ago as a promising, easily accessible, and tunable class of organic photoredox catalysts, but a systematic study on the effect of the electronic nature and of the position of the substituents on both the ground-state and the excited-state redox potentials of these compounds is still lacking. We prepared a set of known functionalized porphyrin derivatives containing different substituents either in one of the meso positions or at a β-pyrrole carbon, and we determined their ground- and (singlet) excited-state redox potentials. We found that while the estimated singlet excited-state energies are essentially unaffected by the introduction of substituents, the redox potentials (both in the ground- and in the singlet excited-state) depend on the electron-withdrawing or electron-donating nature of the substituents. Thus, the presence of groups with electron-withdrawing resonance effects results in an enhancement of the reduction facility of the photocatalyst, both in the ground and in the excited state. We next prepared a second set of four previously unknown meso-substituted porphyrins, having a benzoyl group at different positions. The reduction facility of the porphyrin increases with the proximity of the substituent to the porphine core, reaching a maximum when the benzoyl substituent is introduced at a meso position.
Keywords: photoredox catalysis; photosensitizers; porphyrins; redox potential; substituent effects.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Effect of meso-substituents on the osmium tetraoxide reaction and pinacol-pinacolone rearrangement of the corresponding vic-dihydroxyporphyrins.J Org Chem. 2001 Jun 1;66(11):3930-9. doi: 10.1021/jo0100143. J Org Chem. 2001. PMID: 11375017
-
Characteristic electronic perturbation by asymmetric arrangements of p-aminophenyl substituents in free-base porphyrins.J Phys Chem A. 2014 Jul 10;118(27):4995-5001. doi: 10.1021/jp505072x. Epub 2014 Jun 25. J Phys Chem A. 2014. PMID: 24927474
-
Porphyrin amino acids-amide coupling, redox and photophysical properties of bis(porphyrin) amides.Dalton Trans. 2013 Jul 14;42(26):9727-39. doi: 10.1039/c3dt50711d. Epub 2013 May 17. Dalton Trans. 2013. PMID: 23685531
-
Structural diversity in expanded porphyrins.Acc Chem Res. 2008 Feb;41(2):265-79. doi: 10.1021/ar700091k. Epub 2008 Feb 19. Acc Chem Res. 2008. PMID: 18281947 Review.
-
Porpholactone Chemistry: An Emerging Approach to Bioinspired Photosensitizers with Tunable Near-Infrared Photophysical Properties.Acc Chem Res. 2019 Sep 17;52(9):2620-2633. doi: 10.1021/acs.accounts.9b00119. Epub 2019 Jul 12. Acc Chem Res. 2019. PMID: 31298833 Review.
Cited by
-
Iron Porphyrin-Based Composites for Electrocatalytic Oxygen Reduction Reactions.Molecules. 2024 Nov 29;29(23):5655. doi: 10.3390/molecules29235655. Molecules. 2024. PMID: 39683814 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

