Pirfenidone Alleviates Inflammation and Fibrosis of Acute Respiratory Distress Syndrome by Modulating the Transforming Growth Factor-β/Smad Signaling Pathway
- PMID: 39125585
- PMCID: PMC11311955
- DOI: 10.3390/ijms25158014
Pirfenidone Alleviates Inflammation and Fibrosis of Acute Respiratory Distress Syndrome by Modulating the Transforming Growth Factor-β/Smad Signaling Pathway
Abstract
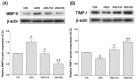
Acute respiratory distress syndrome (ARDS) occurs as an acute onset condition, and patients present with diffuse alveolar damage, refractory hypoxemia, and non-cardiac pulmonary edema. ARDS progresses through an initial exudative phase, an inflammatory phase, and a final fibrotic phase. Pirfenidone, a powerful anti-fibrotic agent, is known as an agent that inhibits the progression of fibrosis in idiopathic pulmonary fibrosis. In this study, we studied the treatment efficiency of pirfenidone on lipopolysaccharide (LPS) and bleomycin-induced ARDS using rats. The ARDS rat model was created by the intratracheal administration of 3 mg/kg LPS of and 3 mg/kg of bleomycin dissolved in 0.2 mL of normal saline. The pirfenidone treatment group was administered 100 or 200 mg/kg of pirfenidone dissolved in 0.5 mL distilled water orally 10 times every 2 days for 20 days. The administration of LPS and bleomycin intratracheally increased lung injury scores and significantly produced pro-inflammatory cytokines. ARDS induction increased the expressions of transforming growth factor (TGF)-β1/Smad-2 signaling factors. Additionally, matrix metalloproteinase (MMP)-9/tissue inhibitor of metalloproteinase (TIMP)-1 imbalance occurred, resulting in enhanced fibrosis-related factors. Treatment with pirfenidone strongly suppressed the expressions of TGF-β1/Smad-2 signaling factors and improved the imbalance of MMP-9/TIMP-1 compared to the untreated group. These effects led to a decrease in fibrosis factors and pro-inflammatory cytokines, promoting the recovery of damaged lung tissue. These results of this study showed that pirfenidone administration suppressed inflammation and fibrosis in the ARDS animal model. Therefore, pirfenidone can be considered a new early treatment for ARDS.
Keywords: acute respiratory distress syndrome; bleomycin; inflammation; lipopolysaccharide; pirfenidone; pulmonary fibrosis.
Conflict of interest statement
Lakkyong Hwang and Jun-Jang Jin are employed by Orient Genia Inc. The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Saguil A., Fargo M. Acute respiratory distress syndrome: Diagnosis and management. Am. Fam. Physician. 2012;85:352–358. - PubMed
-
- Ko I.G., Hwang J.J., Chang B.S., Kim S.H., Jin J.J., Hwang L., Kim C.J., Choi C.W. Polydeoxyribonucleotide ameliorates lipopolysaccharide-induced acute lung injury via modulation of the MAPK/NF-κB signaling pathway in rats. Int. Immunopharmacol. 2020;83:106444. doi: 10.1016/j.intimp.2020.106444. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

