Crosstalk among WEE1 Kinase, AKT, and GSK3 in Nav1.2 Channelosome Regulation
- PMID: 39125637
- PMCID: PMC11311446
- DOI: 10.3390/ijms25158069
Crosstalk among WEE1 Kinase, AKT, and GSK3 in Nav1.2 Channelosome Regulation
Abstract
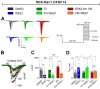
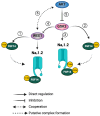
The signaling complex around voltage-gated sodium (Nav) channels includes accessory proteins and kinases crucial for regulating neuronal firing. Previous studies showed that one such kinase, WEE1-critical to the cell cycle-selectively modulates Nav1.2 channel activity through the accessory protein fibroblast growth factor 14 (FGF14). Here, we tested whether WEE1 exhibits crosstalk with the AKT/GSK3 kinase pathway for coordinated regulation of FGF14/Nav1.2 channel complex assembly and function. Using the in-cell split luciferase complementation assay (LCA), we found that the WEE1 inhibitor II and GSK3 inhibitor XIII reduce the FGF14/Nav1.2 complex formation, while the AKT inhibitor triciribine increases it. However, combining WEE1 inhibitor II with either one of the other two inhibitors abolished its effect on the FGF14/Nav1.2 complex formation. Whole-cell voltage-clamp recordings of sodium currents (INa) in HEK293 cells co-expressing Nav1.2 channels and FGF14-GFP showed that WEE1 inhibitor II significantly suppresses peak INa density, both alone and in the presence of triciribine or GSK3 inhibitor XIII, despite the latter inhibitor's opposite effects on INa. Additionally, WEE1 inhibitor II slowed the tau of fast inactivation and caused depolarizing shifts in the voltage dependence of activation and inactivation. These phenotypes either prevailed or were additive when combined with triciribine but were outcompeted when both WEE1 inhibitor II and GSK3 inhibitor XIII were present. Concerted regulation by WEE1 inhibitor II, triciribine, and GSK3 inhibitor XIII was also observed in long-term inactivation and use dependency of Nav1.2 currents. Overall, these findings suggest a complex role for WEE1 kinase-in concert with the AKT/GSK3 pathway-in regulating the Nav1.2 channelosome.
Keywords: fibroblast growth factors; kinase inhibitors; split luciferase assay; voltage-gated sodium channels.
Conflict of interest statement
F.L. is the founder and president of IonTX Inc. However, this activity does not represent a conflict with the present study.
Figures



References
-
- Shavkunov A.S., Wildburger N.C., Nenov M.N., James T.F., Buzhdygan T.P., Panova-Elektronova N.I., Green T.A., Veselenak R.L., Bourne N., Laezza F. The Fibroblast Growth Factor 14: Voltage-gated Sodium Channel Complex Is a New Target of Glycogen Synthase Kinase 3 (GSK3) J. Biol. Chem. 2013;288:19370–19385. doi: 10.1074/jbc.M112.445924. - DOI - PMC - PubMed
-
- Liang L., Fazel Darbandi S., Pochareddy S., Gulden F.O., Gilson M.C., Sheppard B.K., Sahagun A., An J.Y., Werling D.M., Rubenstein J.L.R., et al. Developmental dynamics of voltage-gated sodium channel isoform expression in the human and mouse brain. Genome Med. 2021;13:135. doi: 10.1186/s13073-021-00949-0. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

