The Scavenging Activity of Coenzyme Q10 Plus a Nutritional Complex on Human Retinal Pigment Epithelial Cells
- PMID: 39125641
- PMCID: PMC11311961
- DOI: 10.3390/ijms25158070
The Scavenging Activity of Coenzyme Q10 Plus a Nutritional Complex on Human Retinal Pigment Epithelial Cells
Abstract
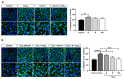
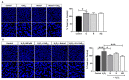
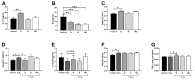
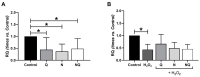
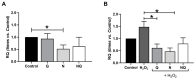
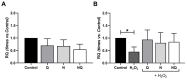
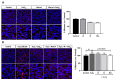
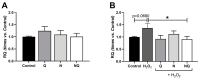
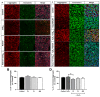
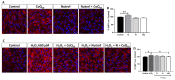
Age-related macular degeneration (AMD) and diabetic retinopathy (DR) are common retinal diseases responsible for most blindness in working-age and elderly populations. Oxidative stress and mitochondrial dysfunction play roles in these pathogenesis, and new therapies counteracting these contributors could be of great interest. Some molecules, like coenzyme Q10 (CoQ10), are considered beneficial to maintain mitochondrial homeostasis and contribute to the prevention of cellular apoptosis. We investigated the impact of adding CoQ10 (Q) to a nutritional antioxidant complex (Nutrof Total®; N) on the mitochondrial status and apoptosis in an in vitro hydrogen peroxide (H2O2)-induced oxidative stress model in human retinal pigment epithelium (RPE) cells. H2O2 significantly increased 8-OHdG levels (p < 0.05), caspase-3 (p < 0.0001) and TUNEL intensity (p < 0.01), and RANTES (p < 0.05), caspase-1 (p < 0.05), superoxide (p < 0.05), and DRP-1 (p < 0.05) levels, and also decreased IL1β, SOD2, and CAT gene expression (p < 0.05) vs. control. Remarkably, Q showed a significant recovery in IL1β gene expression, TUNEL, TNFα, caspase-1, and JC-1 (p < 0.05) vs. H2O2, and NQ showed a synergist effect in caspase-3 (p < 0.01), TUNEL (p < 0.0001), mtDNA, and DRP-1 (p < 0.05). Our results showed that CoQ10 supplementation is effective in restoring/preventing apoptosis and mitochondrial stress-related damage, suggesting that it could be a valid strategy in degenerative processes such as AMD or DR.
Keywords: ARPE-19; DRP-1; age-related macular degeneration (AMD); caspase-3; coenzyme Q10; diabetic retinopathy (DR); mitochondrial stress; oxidative stress.
Conflict of interest statement
A.G.-L. is consultant for Bayer, Novartis, Allergan, Thea, and Roche. The rest of the authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Shankar K., Mehendale H.M. Encyclopedia of Toxicology. 3rd ed. Academic Press; Cambridge, MA, USA: 2014. Oxidative Stress; pp. 735–737. - DOI
-
- Bilbao-Malavé V., González-Zamora J., de la Puente M., Recalde S., Fernandez-Robredo P., Hernandez M., Layana A.G., de Viteri M.S. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Age Related Macular Degeneration, Role in Pathophysiology, and Possible New Therapeutic Strategies. Antioxidants. 2021;10:1170. doi: 10.3390/antiox10081170. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

