The Regulation of Frontal Cortex Cholesterol Metabolism Abnormalities by NR3C1/NRIP1/NR1H2 Is Involved in the Occurrence of Stress-Induced Depression
- PMID: 39125645
- PMCID: PMC11311262
- DOI: 10.3390/ijms25158075
The Regulation of Frontal Cortex Cholesterol Metabolism Abnormalities by NR3C1/NRIP1/NR1H2 Is Involved in the Occurrence of Stress-Induced Depression
Abstract
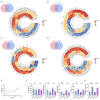
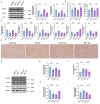
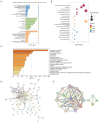
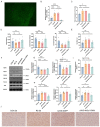
Stress-induced alterations in central neuron metabolism and function are crucial contributors to depression onset. However, the metabolic dysfunctions of the neurons associated with depression and specific molecular mechanisms remain unclear. This study initially analyzed the relationship between cholesterol and depression using the NHANES database. We then induced depressive-like behaviors in mice via restraint stress. Applying bioinformatics, pathology, and molecular biology, we observed the pathological characteristics of brain cholesterol homeostasis and investigated the regulatory mechanisms of brain cholesterol metabolism disorders. Through the NHANES database, we initially confirmed a significant correlation between cholesterol metabolism abnormalities and depression. Furthermore, based on successful stress mouse model establishment, we discovered the number of cholesterol-related DEGs significantly increased in the brain due to stress, and exhibited regional heterogeneity. Further investigation of the frontal cortex, a brain region closely related to depression, revealed stress caused significant disruption to key genes related to cholesterol metabolism, including HMGCR, CYP46A1, ACAT1, APOE, ABCA1, and LDLR, leading to an increase in total cholesterol content and a significant decrease in synaptic proteins PSD-95 and SYN. This indicates cholesterol metabolism affects neuronal synaptic plasticity and is associated with stress-induced depressive-like behavior in mice. Adeno-associated virus interference with NR3C1 in the prefrontal cortex of mice subjected to short-term stress resulted in reduced protein levels of NRIP1, NR1H2, ABCA1, and total cholesterol content. At the same time, it increased synaptic proteins PSD95 and SYN, effectively alleviating depressive-like behavior. Therefore, these results suggest that short-term stress may induce cholesterol metabolism disorders by activating the NR3C1/NRIP1/NR1H2 signaling pathway. This impairs neuronal synaptic plasticity and consequently participates in depressive-like behavior in mice. These findings suggest that abnormal cholesterol metabolism in the brain induced by stress is a significant contributor to depression onset.
Keywords: NR3C1/NRIP1/NR1H2 pathway; cholesterol metabolism; depression; frontal cortex.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Wray N.R., Ripke S., Mattheisen M., Trzaskowski M., Byrne E.M., Abdellaoui A., Adams M.J., Agerbo E., Air T.M., Andlauer T.M.F., et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018;50:668. doi: 10.1038/s41588-018-0090-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

