Insulin-Activated Signaling Pathway and GLUT4 Membrane Translocation in hiPSC-Derived Cardiomyocytes
- PMID: 39125765
- PMCID: PMC11312081
- DOI: 10.3390/ijms25158197
Insulin-Activated Signaling Pathway and GLUT4 Membrane Translocation in hiPSC-Derived Cardiomyocytes
Abstract
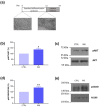
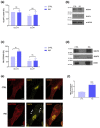
Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) are a cell model now widely used to investigate pathophysiological features of cardiac tissue. Given the invaluable contribution hiPSC-CM could make for studies on cardio-metabolic disorders by defining a postnatal metabolic phenotype, our work herein focused on monitoring the insulin response in CM derived from the hiPSC line UKBi015-B. Western blot analysis on total cell lysates obtained from hiPSC-CM showed increased phosphorylation of both AKT and AS160 following insulin treatment, but failed to highlight any changes in the expression dynamics of the glucose transporter GLUT4. By contrast, the Western blot analysis of membrane fractions, rather than total lysates, revealed insulin-induced plasma membrane translocation of GLUT4, which is known to also occur in postnatal CM. Thus, these findings suggest that hiPSC-derived CMs exhibit an insulin response reminiscent to that of adult CMs regarding intracellular signaling and GLUT4 translocation to the plasma membrane, representing a suitable cellular model in the cardio-metabolic research field. Moreover, our studies also demonstrate the relevance of analyzing membrane fractions rather than total lysates in order to monitor GLUT4 dynamics in response to metabolic regulators in hiPSC-CMs.
Keywords: GLUT4; cardiac maturation; cardiomyocyte; hiPSC-CM; insulin; lactate medium; metabolism; plasma membrane translocation; postnatal cardiac cell.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Bistola V., Nikolopoulou M., Derventzi A., Kataki A., Sfyras N., Nikou N., Toutouza M., Toutouzas P., Stefanadis C., Konstadoulakis M.M. Long-Term Primary Cultures of Human Adult Atrial Cardiac Myocytes: Cell Viability, Structural Properties and BNP Secretion in Vitro. Int. J. Cardiol. 2008;131:113–122. doi: 10.1016/j.ijcard.2007.10.058. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

