A Novel Luciferase-Based Reporter Gene Technology for Simultaneous Optical and Radionuclide Imaging of Cells
- PMID: 39125775
- PMCID: PMC11312113
- DOI: 10.3390/ijms25158206
A Novel Luciferase-Based Reporter Gene Technology for Simultaneous Optical and Radionuclide Imaging of Cells
Abstract
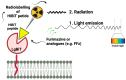
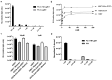
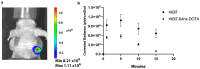
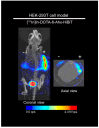
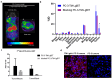
Multimodality reporter gene imaging combines the sensitivity, resolution and translational potential of two or more signals. The approach has not been widely adopted by the animal imaging community, mainly because its utility in this area is unproven. We developed a new complementation-based reporter gene system where the large component of split NanoLuc luciferase (LgBiT) presented on the surface of cells (TM-LgBiT) interacts with a radiotracer consisting of the high-affinity complementary HiBiT peptide labeled with a radionuclide. Radiotracer uptake could be imaged in mice using SPECT/CT and bioluminescence within two hours of implanting reporter-gene-expressing cells. Imaging data were validated by ex vivo biodistribution studies. Following the demonstration of complementation between the TM-LgBiT protein and HiBiT radiotracer, we validated the use of the technology in the highly specific in vivo multimodal imaging of cells. These findings highlight the potential of this new approach to facilitate the advancement of cell and gene therapies from bench to clinic.
Keywords: bioluminescence imaging; luciferase complementation; peptide tracers; radionuclide imaging; reporter gene.
Conflict of interest statement
Authors Thomas A. Kirkland, Mary P. Hall and Lance P. Encell were employed by the company Promega Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

