Skin Aging and the Upcoming Role of Ferroptosis in Geroscience
- PMID: 39125810
- PMCID: PMC11311626
- DOI: 10.3390/ijms25158238
Skin Aging and the Upcoming Role of Ferroptosis in Geroscience
Abstract
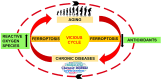
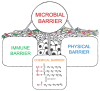
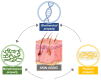
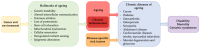
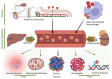
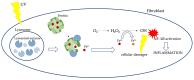
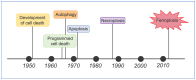
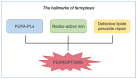
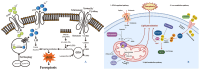
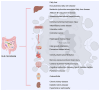
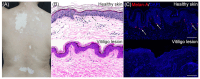
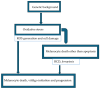
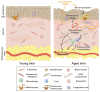
The skin is considered the most important organ system in mammals, and as the population ages, it is important to consider skin aging and anti-aging therapeutic strategies. Exposure of the skin to various insults induces significant changes throughout our lives, differentiating the skin of a young adult from that of an older adult. These changes are caused by a combination of intrinsic and extrinsic aging. We report the interactions between skin aging and its metabolism, showing that the network is due to several factors. For example, iron is an important nutrient for humans, but its level increases with aging, inducing deleterious effects on cellular functions. Recently, it was discovered that ferroptosis, or iron-dependent cell death, is linked to aging and skin diseases. The pursuit of new molecular targets for ferroptosis has recently attracted attention. Prevention of ferroptosis is an effective therapeutic strategy for the treatment of diseases, especially in old age. However, the pathological and biological mechanisms underlying ferroptosis are still not fully understood, especially in skin diseases such as melanoma and autoimmune diseases. Only a few basic studies on regulated cell death exist, and the challenge is to turn the studies into clinical applications.
Keywords: aging; autoimmune diseases; cutaneous diseases; ferroptosis; gut microbiota; melanoma; skin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
NMN recruits GSH to enhance GPX4-mediated ferroptosis defense in UV irradiation induced skin injury.Biochim Biophys Acta Mol Basis Dis. 2022 Jan 1;1868(1):166287. doi: 10.1016/j.bbadis.2021.166287. Epub 2021 Oct 6. Biochim Biophys Acta Mol Basis Dis. 2022. PMID: 34626772
-
Changes in ferrous iron and glutathione promote ferroptosis and frailty in aging Caenorhabditis elegans.Elife. 2020 Jul 21;9:e56580. doi: 10.7554/eLife.56580. Elife. 2020. PMID: 32690135 Free PMC article.
-
The influence of microbiota on ferroptosis in intestinal diseases.Gut Microbes. 2023 Dec;15(2):2263210. doi: 10.1080/19490976.2023.2263210. Epub 2023 Oct 5. Gut Microbes. 2023. PMID: 37795964 Free PMC article. Review.
-
Ferroptosis and aerobic training in ageing.Clin Hemorheol Microcirc. 2024;87(3):347-366. doi: 10.3233/CH-232076. Clin Hemorheol Microcirc. 2024. PMID: 38306027 Review.
-
Ferroptosis: Shedding Light on Mechanisms and Therapeutic Opportunities in Liver Diseases.Cells. 2022 Oct 20;11(20):3301. doi: 10.3390/cells11203301. Cells. 2022. PMID: 36291167 Free PMC article. Review.
Cited by
-
Inflammatory Transformation of Skin Basal Cells as a Key Driver of Cutaneous Aging.Int J Mol Sci. 2025 Mar 14;26(6):2617. doi: 10.3390/ijms26062617. Int J Mol Sci. 2025. PMID: 40141258 Free PMC article.
-
Precision Recovery After Spinal Cord Injury: Integrating CRISPR Technologies, AI-Driven Therapeutics, Single-Cell Omics, and System Neuroregeneration.Int J Mol Sci. 2025 Jul 20;26(14):6966. doi: 10.3390/ijms26146966. Int J Mol Sci. 2025. PMID: 40725213 Free PMC article. Review.
References
-
- Ahmed I.A., Mikail M.A. Anti-Aging Skincare: The Natural and Organic Way. In: Koltover V.K.B.T.-A.-A.P., editor. Anti-Aging Pharmacology. Academic Press; Cambridge, MA, USA: 2023. pp. 269–284.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

