Establishment and Characterization of a Chicken Myoblast Cell Line
- PMID: 39125909
- PMCID: PMC11312951
- DOI: 10.3390/ijms25158340
Establishment and Characterization of a Chicken Myoblast Cell Line
Abstract
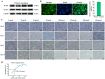
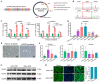
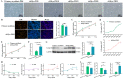
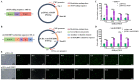
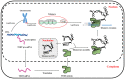
Skeletal muscle, which is predominantly constituted by multinucleated muscle fibers, plays a pivotal role in sustaining bodily movements and energy metabolism. Myoblasts, which serve as precursor cells for differentiation and fusion into muscle fibers, are of critical importance in the exploration of the functional genes associated with embryonic muscle development. However, the in vitro proliferation of primary myoblasts is inherently constrained. In this study, we achieved a significant breakthrough by successfully establishing a chicken myoblast cell line through the introduction of the exogenous chicken telomerase reverse transcriptase (chTERT) gene, followed by rigorous G418-mediated pressure screening. This newly developed cell line, which was designated as chTERT-myoblasts, closely resembled primary myoblasts in terms of morphology and exhibited remarkable stability in culture for at least 20 generations of population doublings without undergoing malignant transformation. In addition, we conducted an exhaustive analysis that encompassed cellular proliferation, differentiation, and transfection characteristics. Our findings revealed that the chTERT-myoblasts had the ability to proliferate, differentiate, and transfect after multiple rounds of population doublings. This achievement not only furnished a valuable source of homogeneous avian cell material for investigating embryonic muscle development, but also provided valuable insights and methodologies for establishing primary cell lines.
Keywords: G418 screening; chTERT; chicken; myoblast cell line; proliferation and differentiation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Male-Biased gga-miR-2954 Regulates Myoblast Proliferation and Differentiation of Chicken Embryos by Targeting YY1.Genes (Basel). 2021 Aug 27;12(9):1325. doi: 10.3390/genes12091325. Genes (Basel). 2021. PMID: 34573307 Free PMC article.
-
The transient expression of miR-203 and its inhibiting effects on skeletal muscle cell proliferation and differentiation.Cell Death Dis. 2014 Jul 17;5(7):e1347. doi: 10.1038/cddis.2014.289. Cell Death Dis. 2014. PMID: 25032870 Free PMC article.
-
METTL16 inhibits differentiation and promotes proliferation and slow myofibers formation in chicken myoblasts.Poult Sci. 2024 Dec;103(12):104384. doi: 10.1016/j.psj.2024.104384. Epub 2024 Oct 5. Poult Sci. 2024. PMID: 39418792 Free PMC article.
-
Regulation of Skeletal Muscle Myoblast Differentiation and Proliferation by Pannexins.Adv Exp Med Biol. 2017;925:57-73. doi: 10.1007/5584_2016_53. Adv Exp Med Biol. 2017. PMID: 27518505 Review.
-
Mechanisms regulating myoblast fusion: A multilevel interplay.Semin Cell Dev Biol. 2020 Aug;104:81-92. doi: 10.1016/j.semcdb.2020.02.004. Epub 2020 Feb 13. Semin Cell Dev Biol. 2020. PMID: 32063453 Review.
Cited by
-
From Development to Regeneration: Insights into Flight Muscle Adaptations from Bat Muscle Cell Lines.Cells. 2025 Aug 1;14(15):1190. doi: 10.3390/cells14151190. Cells. 2025. PMID: 40801622 Free PMC article.
-
Chicken CircZNF609 encodes a protein induced by IRES-like region that inhibits the proliferation and promotes the differentiation of myoblasts.Poult Sci. 2025 Aug;104(8):105339. doi: 10.1016/j.psj.2025.105339. Epub 2025 May 26. Poult Sci. 2025. PMID: 40482626 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 31972550/National Natural Science Foundation of China
- 2020B1515420008 and 2023A1515011057/Natural Science Foundation of Guangdong Province
- 060302052104/Program for Scientific Research Start-up Funds of Guangdong Ocean University
- 2023A1515110023/Guangdong Province Basic and Applied Basic Research Regional Joint Fund-Youth Fund Project
LinkOut - more resources
Full Text Sources

