Copper(II) and Zinc(II) Complexes with Bacterial Prodigiosin Are Targeting Site III of Bovine Serum Albumin and Acting as DNA Minor Groove Binders
- PMID: 39125963
- PMCID: PMC11313072
- DOI: 10.3390/ijms25158395
Copper(II) and Zinc(II) Complexes with Bacterial Prodigiosin Are Targeting Site III of Bovine Serum Albumin and Acting as DNA Minor Groove Binders
Abstract
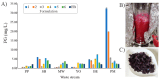
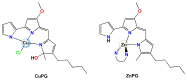
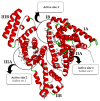
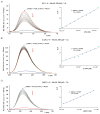
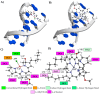
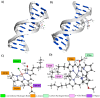
The negative environmental and social impacts of food waste accumulation can be mitigated by utilizing bio-refineries' approach where food waste is revalorized into high-value products, such as prodigiosin (PG), using microbial bioprocesses. The diverse biological activities of PG position it as a promising compound, but its high production cost and promiscuous bioactivity hinder its wide application. Metal ions can modulate the electronic properties of organic molecules, leading to novel mechanisms of action and increased target potency, while metal complex formation can improve the stability, solubility and bioavailability of the parent compound. The objectives of this study were optimizing PG production through bacterial fermentation using food waste, allowing good quantities of the pure natural product for further synthesizing and evaluating copper(II) and zinc(II) complexes with it. Their antimicrobial and anticancer activities were assessed, and their binding affinity toward biologically important molecules, bovine serum albumin (BSA) and DNA was investigated by fluorescence emission spectroscopy and molecular docking. The yield of 83.1 mg/L of pure PG was obtained when processed meat waste at 18 g/L was utilized as the sole fermentation substrate. The obtained complexes CuPG and ZnPG showed high binding affinity towards target site III of BSA, and molecular docking simulations highlighted the affinity of the compounds for DNA minor grooves.
Keywords: DNA interactions; Serratia marcescens; bio-refinery; metal complexation; prodigiosin; protein interactions; waste.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gustavsson J., Cederberg C., Sonesson U., van Otterdijk R., Meybeck A. Global Food Losses and Food Waste: Extent, Causes and Prevention. Food and Agriculture Organization of the United Nations; Rome, Italy: 2011.
-
- Venil C.K., Zakaria Z.A., Ahmad W.A. Bacterial pigments and their applications. Process Biochem. 2013;48:1065–1079. doi: 10.1016/j.procbio.2013.06.006. - DOI
MeSH terms
Substances
Grants and funding
- 7730810/Science Fund of the Republic of Serbia
- 451-03-66/2024-03/200042/Ministry of Science, Technological Development and Innovations of the Republic of Serbia
- 451-03-65/2024-03/200122/Ministry of Science, Technological Development and Innovations of the Republic of Serbia
- 451-03-66/2024-03/200122/Ministry of Science, Technological Development and Innovations of the Republic of Serbia
- 451-03-66/2024-03/200378/Ministry of Science, Technological Development and Innovations of the Republic of Serbia
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

