Targeting Myeloid Differentiation Primary Response Protein 88 (MyD88) and Galectin-3 to Develop Broad-Spectrum Host-Mediated Therapeutics against SARS-CoV-2
- PMID: 39125989
- PMCID: PMC11313481
- DOI: 10.3390/ijms25158421
Targeting Myeloid Differentiation Primary Response Protein 88 (MyD88) and Galectin-3 to Develop Broad-Spectrum Host-Mediated Therapeutics against SARS-CoV-2
Abstract
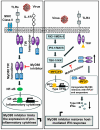
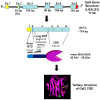
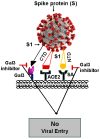
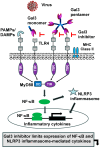
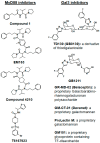
Nearly six million people worldwide have died from the coronavirus disease (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Although COVID-19 vaccines are largely successful in reducing the severity of the disease and deaths, the decline in vaccine-induced immunity over time and the continuing emergence of new viral variants or mutations underscore the need for an alternative strategy for developing broad-spectrum host-mediated therapeutics against SARS-CoV-2. A key feature of severe COVID-19 is dysregulated innate immune signaling, culminating in a high expression of numerous pro-inflammatory cytokines and chemokines and a lack of antiviral interferons (IFNs), particularly type I (alpha and beta) and type III (lambda). As a natural host defense, the myeloid differentiation primary response protein, MyD88, plays pivotal roles in innate and acquired immune responses via the signal transduction pathways of Toll-like receptors (TLRs), a type of pathogen recognition receptors (PRRs). However, recent studies have highlighted that infection with viruses upregulates MyD88 expression and impairs the host antiviral response by negatively regulating type I IFN. Galectin-3 (Gal3), another key player in viral infections, has been shown to modulate the host immune response by regulating viral entry and activating TLRs, the NLRP3 inflammasome, and NF-κB, resulting in the release of pro-inflammatory cytokines and contributing to the overall inflammatory response, the so-called "cytokine storm". These studies suggest that the specific inhibition of MyD88 and Gal3 could be a promising therapy for COVID-19. This review presents future directions for MyD88- and Gal3-targeted antiviral drug discovery, highlighting the potential to restore host immunity in SARS-CoV-2 infections.
Keywords: COVID-19; IFNs; MyD88; NLRP3; PRR; SARS-CoV-2; TLRs; cytokine; galectin-3.
Conflict of interest statement
H.A., K.A. and S.S. own GlycoMantra equity. GlycoMantra owns a patent (pending) on the combination of MyD88 inhibitor and Gal3 inhibitor for the therapy of respiratory viral infections, including SARS-CoV-2. The authors declare no conflicts of interest.
Figures
Similar articles
-
Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2.mBio. 2020 Sep 10;11(5):e01928-20. doi: 10.1128/mBio.01928-20. mBio. 2020. PMID: 32913009 Free PMC article.
-
TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines.Nat Immunol. 2021 Jul;22(7):829-838. doi: 10.1038/s41590-021-00937-x. Epub 2021 May 7. Nat Immunol. 2021. PMID: 33963333 Free PMC article.
-
MyD88 and beyond: a perspective on MyD88-targeted therapeutic approach for modulation of host immunity.Immunol Res. 2021 Apr;69(2):117-128. doi: 10.1007/s12026-021-09188-2. Epub 2021 Apr 8. Immunol Res. 2021. PMID: 33834387 Free PMC article. Review.
-
Toll-Like Receptor 3 Signaling via TRIF Contributes to a Protective Innate Immune Response to Severe Acute Respiratory Syndrome Coronavirus Infection.mBio. 2015 May 26;6(3):e00638-15. doi: 10.1128/mBio.00638-15. mBio. 2015. PMID: 26015500 Free PMC article.
-
Innate Immune Signaling and Proteolytic Pathways in the Resolution or Exacerbation of SARS-CoV-2 in Covid-19: Key Therapeutic Targets?Front Immunol. 2020 May 28;11:1229. doi: 10.3389/fimmu.2020.01229. eCollection 2020. Front Immunol. 2020. PMID: 32574272 Free PMC article. Review.
Cited by
-
Administration of a Recombinant Fusion Protein of IFN-γ and CD154 Inhibited the Infection of Chicks with Salmonella enterica.Vet Sci. 2025 Feb 2;12(2):112. doi: 10.3390/vetsci12020112. Vet Sci. 2025. PMID: 40005871 Free PMC article.
-
Structural and Functional Characteristics of TLR19 in Barbel Chub Compared to TLR19 in Grass Carp.Int J Mol Sci. 2025 Mar 27;26(7):3103. doi: 10.3390/ijms26073103. Int J Mol Sci. 2025. PMID: 40243814 Free PMC article.
-
HIV, Inflammation, and Immunometabolism: A Model of the Inflammatory Theory of Disease.Viruses. 2025 Jun 11;17(6):839. doi: 10.3390/v17060839. Viruses. 2025. PMID: 40573430 Free PMC article. Review.
References
-
- Hatton C.F., Botting R.A., Dueñas M.E., Haq I.J., Verdon B., Thompson B.J., Spegarova J.S., Gothe F., Stephenson E., Gardner A.I., et al. Delayed induction of type I and III interferons mediates nasal epithelial cell permissiveness to SARS-CoV-2. Nat. Commun. 2021;12:7092. doi: 10.1038/s41467-021-27318-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

