Arylphthalide Delays Diabetic Retinopathy via Immunomodulating the Early Inflammatory Response in an Animal Model of Type 1 Diabetes Mellitus
- PMID: 39126007
- PMCID: PMC11313200
- DOI: 10.3390/ijms25158440
Arylphthalide Delays Diabetic Retinopathy via Immunomodulating the Early Inflammatory Response in an Animal Model of Type 1 Diabetes Mellitus
Abstract
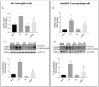
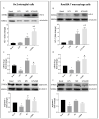
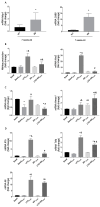
Diabetic retinopathy (DR) is one of the most prevalent secondary complications associated with diabetes. Specifically, Type 1 Diabetes Mellitus (T1D) has an immune component that may determine the evolution of DR by compromising the immune response of the retina, which is mediated by microglia. In the early stages of DR, the permeabilization of the blood-retinal barrier allows immune cells from the peripheral system to interact with the retinal immune system. The use of new bioactive molecules, such as 3-(2,4-dihydroxyphenyl)phthalide (M9), with powerful anti-inflammatory activity, might represent an advance in the treatment of diseases like DR by targeting the immune systems responsible for its onset and progression. Our research aimed to investigate the molecular mechanisms involved in the interaction of specific cells of the innate immune system during the progression of DR and the reduction in inflammatory processes contributing to the pathology. In vitro studies were conducted exposing Bv.2 microglial and Raw264.7 macrophage cells to proinflammatory stimuli for 24 h, in the presence or absence of M9. Ex vivo and in vivo approaches were performed in BB rats, an animal model for T1D. Retinal explants from BB rats were cultured with M9. Retinas from BB rats treated for 15 days with M9 via intraperitoneal injection were analyzed to determine survival, cellular signaling, and inflammatory markers using qPCR, Western blot, or immunofluorescence approaches. Retinal structure images were acquired via Spectral-Domain-Optical Coherence Tomography (SD-OCT). Our results show that the treatment with M9 significantly reduces inflammatory processes in in vitro, ex vivo, and in vivo models of DR. M9 works by inhibiting the proinflammatory responses during DR progression mainly affecting immune cell responses. It also induces an anti-inflammatory response, primarily mediated by microglial cells, leading to the synthesis of Arginase-1 and Hemeoxygenase-1(HO-1). Ultimately, in vivo administration of M9 preserves the retinal integrity from the degeneration associated with DR progression. Our findings demonstrate a specific interaction between both retinal and systemic immune cells in the progression of DR, with a differential response to treatment, mainly driven by microglia in the anti-inflammatory action. In vivo treatment with M9 induces a switch in immune cell phenotypes and functions that contributes to delaying the DR progression, positioning microglial cells as a new and specific therapeutic target in DR.
Keywords: HO1; M2 response; arylphthalides; diabetic retinopathy; immunomodulation; inflammation; microglia; type 1 diabetes mellitus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

