Molecular Pro-Apoptotic Activities of Flavanone Derivatives in Cyclodextrin Complexes: New Implications for Anticancer Therapy
- PMID: 39126058
- PMCID: PMC11312998
- DOI: 10.3390/ijms25158488
Molecular Pro-Apoptotic Activities of Flavanone Derivatives in Cyclodextrin Complexes: New Implications for Anticancer Therapy
Abstract
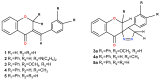
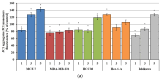
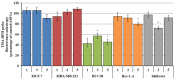
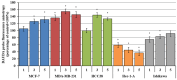
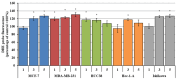
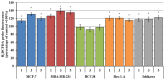
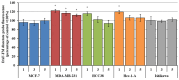
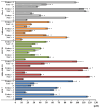
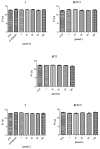
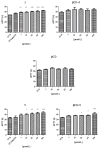
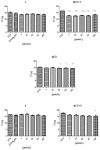
This study evaluates the antiproliferative potential of flavanones, chromanones and their spiro-1-pyrazoline derivatives as well as their inclusion complexes. The main goal was to determine the biological basis of molecular pro-apoptotic activities and the participation of reactive oxygen species (ROS) in shaping the cytotoxic properties of the tested conjugates. For this purpose, changes in mitochondrial potential and the necrotic/apoptotic cell fraction were analyzed. Testing with specific fluorescent probes found that ROS generation had a significant contribution to the biological anticancer activity of complexes of flavanone analogues. TT (thrombin time), PT (prothrombin time) and APTT (activated partial tromboplastin time) were used to evaluate the influence of the compounds on the extrinsic and intrinsic coagulation pathway. Hemolysis assays and microscopy studies were conducted to determine the effect of the compounds on RBCs.
Keywords: anticancer properties; biocompatibility; crystal structure; cyclodextrines; docking studies; flavonoid derivatives; inclusion complexes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Evaluation of In Vitro Biological Activity of Flavanone/Chromanone Derivatives: Molecular Analysis of Anticancer Mechanisms in Colorectal Cancer.Int J Mol Sci. 2024 Dec 3;25(23):12985. doi: 10.3390/ijms252312985. Int J Mol Sci. 2024. PMID: 39684693 Free PMC article.
-
The synthetic flavanone 6-methoxy-2-(naphthalen-1-yl)chroman-4-one induces apoptosis and activation of the MAPK pathway in human U-937 leukaemia cells.Bioorg Chem. 2020 Jan;94:103450. doi: 10.1016/j.bioorg.2019.103450. Epub 2019 Nov 21. Bioorg Chem. 2020. PMID: 31785856
-
Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest.PLoS One. 2014 Oct 16;9(10):e110003. doi: 10.1371/journal.pone.0110003. eCollection 2014. PLoS One. 2014. PMID: 25330158 Free PMC article.
-
Investigation of the mechanism and apoptotic pathway induced by 4β cinnamido linked podophyllotoxins against human lung cancer cells A549.Apoptosis. 2015 Nov;20(11):1518-29. doi: 10.1007/s10495-015-1173-6. Apoptosis. 2015. PMID: 26386574
-
Novel pyrazolo[3,4-d]pyrimidine derivatives inhibit human cancer cell proliferation and induce apoptosis by ROS generation.Arch Pharm (Weinheim). 2020 Apr;353(4):e1900296. doi: 10.1002/ardp.201900296. Epub 2020 Feb 19. Arch Pharm (Weinheim). 2020. PMID: 32073686
Cited by
-
Special Issue "Advances in Drug Discovery and Synthesis".Int J Mol Sci. 2025 Jan 11;26(2):584. doi: 10.3390/ijms26020584. Int J Mol Sci. 2025. PMID: 39859300 Free PMC article.
References
-
- Jicsinszky L., Fenyvesi E., Hashimoto H., Ueno A. Chapter 4. Cyclodextrin derivatives. Cyclodextrins. In: Szejtli J., Osa T., editors. Comprehensive Supramolecular Chemistry. Pergamon; Oxford, UK: 1996. pp. 57–188.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

