Connexin 43 Modulation in Human Chondrocytes, Osteoblasts and Cartilage Explants: Implications for Inflammatory Joint Disorders
- PMID: 39126115
- PMCID: PMC11313680
- DOI: 10.3390/ijms25158547
Connexin 43 Modulation in Human Chondrocytes, Osteoblasts and Cartilage Explants: Implications for Inflammatory Joint Disorders
Abstract
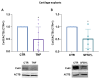
Connexin 43 (Cx43) is crucial for the development and homeostasis of the musculoskeletal system, where it plays multifaceted roles, including intercellular communication, transcriptional regulation and influencing osteogenesis and chondrogenesis. Here, we investigated Cx43 modulation mediated by inflammatory stimuli involved in osteoarthritis, i.e., 10 ng/mL Tumor Necrosis Factor alpha (TNFα) and/or 1 ng/mL Interleukin-1 beta (IL-1β), in primary chondrocytes (CH) and osteoblasts (OB). Additionally, we explored the impact of synovial fluids from osteoarthritis patients in CH and cartilage explants, providing a more physio-pathological context. The effect of TNFα on Cx43 expression in cartilage explants was also assessed. TNFα downregulated Cx43 levels both in CH and OB (-73% and -32%, respectively), while IL-1β showed inconclusive effects. The reduction in Cx43 levels was associated with a significant downregulation of the coding gene GJA1 expression in OB only (-65%). The engagement of proteasome in TNFα-induced effects, already known in CH, was also observed in OB. TNFα treatment significantly decreased Cx43 expression also in cartilage explants. Of note, Cx43 expression was halved by synovial fluid in both CH and cartilage explants. This study unveils the regulation of Cx43 in diverse musculoskeletal cell types under various stimuli and in different contexts, providing insights into its modulation in inflammatory joint disorders.
Keywords: Connexin 43; IL-1β; TNFα; cartilage explants; chondrocytes; osteoarthritis; osteoblasts; synovial fluid.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

