Anatomical and physiological responses of roots and rhizomes in Oryza longistaminata to soil water gradients
- PMID: 39126169
- PMCID: PMC12682861
- DOI: 10.1093/aob/mcae131
Anatomical and physiological responses of roots and rhizomes in Oryza longistaminata to soil water gradients
Abstract
Background and aims: Roots and rhizomes are crucial for the adaptation of clonal plants to soil water gradients. Oryza longistaminata, a rhizomatous wild rice, is of particular interest for perennial rice breeding owing to its resilience in abiotic stress conditions. Although root responses to soil flooding are well studied, rhizome responses to water gradients remain underexplored. We hypothesize that physiological integration of Oryza longistaminata mitigates heterogeneous water-deficit stress through interconnected rhizomes, and both roots and rhizomes respond to contrasting water conditions.
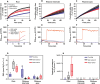
Methods: We investigated the physiological integration between mother plants and ramets, measuring key photosynthetic parameters (photosynthetic and transpiration rates and stomatal conductance) using an infrared gas analyser. Moreover, root and rhizome responses to three water regimes (flooding, well watered and water deficit) were examined by measuring radial water loss and apparent permeance to O2, along with histochemical and anatomical characterization.
Key results: Our experiment highlights the role of physiological integration via interconnected rhizomes in mitigating water-deficit stress. Severing rhizome connections from mother plants or ramets exposed to water-deficit conditions led to significant decreases in key photosynthetic parameters, underscoring the importance of rhizome connections in bidirectional stress mitigation. Additionally, O. longistaminata rhizomes exhibited constitutive suberized and lignified apoplastic barriers, and such barriers were induced in roots in water stress. Anatomically, both rhizomes and roots respond in a similar manner to water gradients, showing smaller diameters in water-deficit conditions and larger diameters in flooding conditions.
Conclusion: Our findings indicate that physiological integration through interconnected rhizomes helps to alleviate water-deficit stress when either the mother plant or the ramet is experiencing water deficit, while the counterpart is in control conditions. Moreover, O. longistaminata can adapt to various soil water regimes by regulating anatomical and physiological traits of roots and rhizomes.
Keywords: Apparent permeance to O2; cortex to stele ratio; drought; flooding; large gas spaces; number of vascular bundles; perennial rice; physiological integration; radial water loss; red rice; root porosity; tissue diameter.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Annals of Botany Company.
Conflict of interest statement
None declared.
Figures




References
-
- Armstrong J, Armstrong W, Beckett PM. 1992. Phragmites australis: venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytologist 120: 197–207.
-
- Armstrong J, Afreen-Zobayed F, Blyth S, Armstrong W. 1999. Phragmites australis: effects of shoot submergence on seedling growth and survival and radial oxygen loss from roots. Aquatic Botany 64: 275–289.
-
- Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM. 2000. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Annals of Botany 86: 687–703.
-
- Azhiri-Sigari T, Yamauchi A, Kamoshita A, Wade LJ. 2000. Genotypic variation in response of rainfed lowland rice to drought and rewatering: II. Root growth. Plant Production Science 3: 180–188.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

