Type I interferon governs immunometabolic checkpoints that coordinate inflammation during Staphylococcal infection
- PMID: 39126652
- PMCID: PMC11590196
- DOI: 10.1016/j.celrep.2024.114607
Type I interferon governs immunometabolic checkpoints that coordinate inflammation during Staphylococcal infection
Abstract
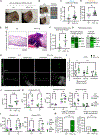
Macrophage metabolic plasticity is central to inflammatory programming, yet mechanisms of coordinating metabolic and inflammatory programs during infection are poorly defined. Here, we show that type I interferon (IFN) temporally guides metabolic control of inflammation during methicillin-resistant Staphylococcus aureus (MRSA) infection. We find that staggered Toll-like receptor and type I IFN signaling in macrophages permit a transient energetic state of combined oxidative phosphorylation (OXPHOS) and aerobic glycolysis followed by inducible nitric oxide synthase (iNOS)-mediated OXPHOS disruption. This disruption promotes type I IFN, suppressing other pro-inflammatory cytokines, notably interleukin-1β. Upon infection, iNOS expression peaks at 24 h, followed by lactate-driven Nos2 repression via histone lactylation. Type I IFN pre-conditioning prolongs infection-induced iNOS expression, amplifying type I IFN. Cutaneous MRSA infection in mice constitutively expressing epidermal type I IFN results in elevated iNOS levels, impaired wound healing, vasculopathy, and lung infection. Thus, kinetically regulated type I IFN signaling coordinates immunometabolic checkpoints that control infection-induced inflammation.
Keywords: CP: Immunology; CP: Metabolism; Staphylococcus aureus; epigenetics; immunometabolism; inflammation; innate immunity; interferon; lactate; macrophage; nitric oxide; respiratory complex.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.A.L. has received consulting fees from Astellas Pharmaceuticals, Odyssey Therapeutics, and T-Knife Therapeutics and is an inventor on patents pertaining to Kras-regulated metabolic pathways, redox control pathways in pancreatic cancer, and targeting the GOT1 pathway as a therapeutic approach (US patent no. 2015126580-A1, May 7, 2015; US patent no. 20190136238, May 9, 2019; and international patent no. WO2013177426-A2, April 23, 2015). J.M.K. has received grant support from Q32 Bio, Celgene/BMS, Ventus Therapeutics, ROME Therapeutics, and Janssen. J.M.K. has served on advisory boards for AstraZeneca, Eli Lilly, GlaxoSmithKline, Gilead, Bristol Myers Squibb, Avion Pharmaceuticals, Provention Bio, Aurinia Pharmaceuticals, Ventus Therapeutics, and ROME Therapeutics.
Figures


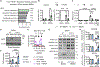


References
-
- Tandon P, Abrams ND, Carrick DM, Chander P, Dwyer J, Fuldner R, Gannot G, Laughlin M, McKie G, PrabhuDas M, et al. (2021). Metabolic Regulation of Inflammation and Its Resolution: Current Status, Clinical Needs, Challenges, and Opportunities. J. Immunol. 207, 2625–2630. 10.4049/jimmunol.2100829. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

