Integrative proteomics identifies a conserved Aβ amyloid responsome, novel plaque proteins, and pathology modifiers in Alzheimer's disease
- PMID: 39127040
- PMCID: PMC11384960
- DOI: 10.1016/j.xcrm.2024.101669
Integrative proteomics identifies a conserved Aβ amyloid responsome, novel plaque proteins, and pathology modifiers in Alzheimer's disease
Abstract
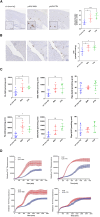
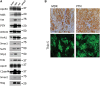
Alzheimer's disease (AD) is a complex neurodegenerative disorder that develops over decades. AD brain proteomics reveals vast alterations in protein levels and numerous altered biologic pathways. Here, we compare AD brain proteome and network changes with the brain proteomes of amyloid β (Aβ)-depositing mice to identify conserved and divergent protein networks with the conserved networks identifying an Aβ amyloid responsome. Proteins in the most conserved network (M42) accumulate in plaques, cerebrovascular amyloid (CAA), and/or dystrophic neuronal processes, and overexpression of two M42 proteins, midkine (Mdk) and pleiotrophin (PTN), increases the accumulation of Aβ in plaques and CAA. M42 proteins bind amyloid fibrils in vitro, and MDK and PTN co-accumulate with cardiac transthyretin amyloid. M42 proteins appear intimately linked to amyloid deposition and can regulate amyloid deposition, suggesting that they are pathology modifiers and thus putative therapeutic targets. We posit that amyloid-scaffolded accumulation of numerous M42+ proteins is a central mechanism mediating downstream pathophysiology in AD.
Keywords: Alzheimer’s disease; Midkine; Pleiotrophin; aggregation; amyloid; animal models; plaques; proteomics.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






Update of
-
Aβ Amyloid Scaffolds the Accumulation of Matrisome and Additional Proteins in Alzheimer's Disease.bioRxiv [Preprint]. 2023 Dec 7:2023.11.29.568318. doi: 10.1101/2023.11.29.568318. bioRxiv. 2023. Update in: Cell Rep Med. 2024 Aug 20;5(8):101669. doi: 10.1016/j.xcrm.2024.101669. PMID: 38076912 Free PMC article. Updated. Preprint.
References
-
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Sims J.R., Zimmer J.A., Evans C.D., Lu M., Ardayfio P., Sparks J., Wessels A.M., Shcherbinin S., Wang H., Monkul Nery E.S., et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. 2023;330:512–527. doi: 10.1001/jama.2023.13239. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

