The Velvet transcription factor PnVeA regulates necrotrophic effectors and secondary metabolism in the wheat pathogen Parastagonospora nodorum
- PMID: 39127645
- PMCID: PMC11316297
- DOI: 10.1186/s12866-024-03454-7
The Velvet transcription factor PnVeA regulates necrotrophic effectors and secondary metabolism in the wheat pathogen Parastagonospora nodorum
Abstract
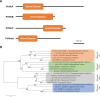
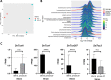
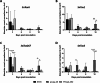
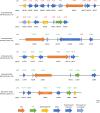
The fungus Parastagonospora nodorum causes septoria nodorum blotch on wheat. The role of the fungal Velvet-family transcription factor VeA in P. nodorum development and virulence was investigated here. Deletion of the P. nodorum VeA ortholog, PnVeA, resulted in growth abnormalities including pigmentation, abolished asexual sporulation and highly reduced virulence on wheat. Comparative RNA-Seq and RT-PCR analyses revealed that the deletion of PnVeA also decoupled the expression of major necrotrophic effector genes. In addition, the deletion of PnVeA resulted in an up-regulation of four predicted secondary metabolite (SM) gene clusters. Using liquid-chromatography mass-spectrometry, it was observed that one of the SM gene clusters led to an accumulation of the mycotoxin alternariol. PnVeA is essential for asexual sporulation, full virulence, secondary metabolism and necrotrophic effector regulation.
Keywords: Parastagonospora nodorum; Necrotrophic effector; Secondary metabolite; Velvet A; Wheat.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

