High-affinity biomolecular interactions are modulated by low-affinity binders
- PMID: 39127695
- PMCID: PMC11316740
- DOI: 10.1038/s41540-024-00410-z
High-affinity biomolecular interactions are modulated by low-affinity binders
Abstract
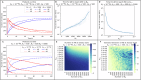
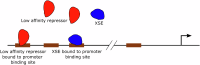
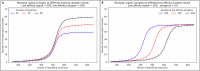
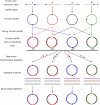
The strength of molecular interactions is characterized by their dissociation constants (KD). Only high-affinity interactions (KD ≤ 10-8 M) are extensively investigated and support binary on/off switches. However, such analyses have discounted the presence of low-affinity binders (KD > 10-5 M) in the cellular environment. We assess the potential influence of low-affinity binders on high-affinity interactions. By employing Gillespie stochastic simulations and continuous methods, we demonstrate that the presence of low-affinity binders can alter the kinetics and the steady state of high-affinity interactions. We refer to this effect as 'herd regulation' and have evaluated its possible impact in two different contexts including sex determination in Drosophila melanogaster and in signalling systems that employ molecular thresholds. We have also suggested experiments to validate herd regulation in vitro. We speculate that low-affinity binders are prevalent in biological contexts where the outcomes depend on molecular thresholds impacting homoeostatic regulation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hu, W.-S. Kinetics of biochemical reactions. In Engineering Principles in Biotechnology (ed. Hu, W.-S.) 95–144 (Wiley, 2017).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

