Recurrent adamantinomatous craniopharyngiomas show MAPK pathway activation, clonal evolution and rare TP53-loss-mediated malignant progression
- PMID: 39127699
- PMCID: PMC11316312
- DOI: 10.1186/s40478-024-01838-4
Recurrent adamantinomatous craniopharyngiomas show MAPK pathway activation, clonal evolution and rare TP53-loss-mediated malignant progression
Abstract
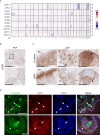
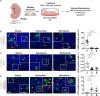
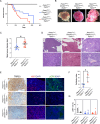
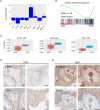
The two types of craniopharyngioma, adamantinomatous (ACP) and papillary (PCP), are clinically relevant tumours in children and adults. Although the biology of primary craniopharyngioma is starting to be unravelled, little is known about the biology of recurrence. To fill this gap in knowledge, we have analysed through methylation array, RNA sequencing and pERK1/2 immunohistochemistry a cohort of paired primary and recurrent samples (32 samples from 14 cases of ACP and 4 cases of PCP). We show the presence of copy number alterations and clonal evolution across recurrence in 6 cases of ACP, and analysis of additional whole genome sequencing data from the Children's Brain Tumour Network confirms chromosomal arm copy number changes in at least 7/67 ACP cases. The activation of the MAPK/ERK pathway, a feature previously shown in primary ACP, is observed in all but one recurrent cases of ACP. The only ACP without MAPK activation is an aggressive case of recurrent malignant human craniopharyngioma harbouring a CTNNB1 mutation and loss of TP53. Providing support for a functional role of this TP53 mutation, we show that Trp53 loss in a murine model of ACP results in aggressive tumours and reduced mouse survival. Finally, we characterise the tumour immune infiltrate showing differences in the cellular composition and spatial distribution between ACP and PCP. Together, these analyses have revealed novel insights into recurrent craniopharyngioma and provided preclinical evidence supporting the evaluation of MAPK pathway inhibitors and immunomodulatory approaches in clinical trials in against recurrent ACP.
Keywords: Craniopharyngioma; MAPK signalling pathway; MEK inhibitor; Macrophage/microglia.
© 2024. The Author(s).
Conflict of interest statement
Cassie Kline receives clinical trial support and drug supply from Bristol-Myers Squibb Co and Day One Biotherapeutics for the PNOC029 study (NCT05465174). In addition, she has other contracts relevant to other clinical trials not pertinent to this publication, specifically Curis Inc, Regeneron Pharmaceuticals, Midatech, Ipsen, Chimerix and Kazia.
Figures

References
-
- Goschzik T, Gessi M, Dreschmann V et al (2017) Genomic alterations of adamantinomatous and papillary craniopharyngioma. J Neuropathol Exp Neurol 76(2):126–134 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

