The Involvement of the Serotonergic System in Ketamine and Fluoxetine Combination-induced Cognitive Impairments in Mice
- PMID: 39128082
- PMCID: PMC11332274
- DOI: 10.5152/eurasianjmed.2024.23219
The Involvement of the Serotonergic System in Ketamine and Fluoxetine Combination-induced Cognitive Impairments in Mice
Abstract
Background: Glutamatergic N-methyl-D-aspartate (NMDA) receptors play vital roles in memory formation. Changes in the activity of these receptors influence memory processes. Ketamine is a noncompetitive NMDA receptor antagonist drug with promising mood-altering and pain-reducing effects in low doses. These effects are believed to be related to altered serotonergic transmission.
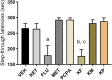
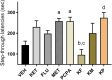
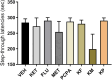
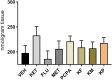
Methods: The present study investigated the involvement of the serotonergic system in low-dose ketamine administrations' effects on memory acquisition, consolidation, and retrieval processes. Sixty-four male BALB/c mice were used in this experiment and separated into 8t groups. Mice were treated subchronically with a selective serotonin reuptake inhibitor, fluoxetine, and a serotonin depletion agent, p-chlorophenylalanine (pCPA). A serotonin antagonist, methiothepin, and ketamine were acutely administered 60 minutes before or after the behavioral tests. A passive avoidance (PA) test measured emotional memory acquisition, consolidation, and retrieval processes. Hippocampi malondialdehyde (MDA) levels were analyzed, and histopathological examinations were performed.
Results: Ketamine alone did not significantly affect memory encoding processes in the PA test, while the ketamine-fluoxetine combination disrupted memory consolidation. Fluoxetine negatively affected the memory acquisition process, which was normalized during the consolidation and retrieval trials. Drug applications did not significantly alter hippocampal MDA levels. In all ketamine-applied groups, histopathologic alterations were evident.
Conclusion: Low-dose ketamine administration induces neurodegeneration, and it also impairs memory functions when combined with fluoxetine, indicating increased serotonergic transmission may be involved in the memory-impairing and neurotoxic effects of ketamine.
Conflict of interest statement
Figures

Similar articles
-
Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice.Neuropharmacology. 2017 Jan;112(Pt A):198-209. doi: 10.1016/j.neuropharm.2016.05.010. Epub 2016 May 17. Neuropharmacology. 2017. PMID: 27211253
-
Ketamine affects memory consolidation: differential effects in T-maze and passive avoidance paradigms in mice.Neuroscience. 2006 Jul 7;140(3):993-1002. doi: 10.1016/j.neuroscience.2006.02.062. Neuroscience. 2006. PMID: 16600517
-
A single dose of vortioxetine, but not ketamine or fluoxetine, increases plasticity-related gene expression in the rat frontal cortex.Eur J Pharmacol. 2016 Sep 5;786:29-35. doi: 10.1016/j.ejphar.2016.05.029. Epub 2016 May 25. Eur J Pharmacol. 2016. PMID: 27235984
-
[Glutaminergic hypothesis of schizophrenia: clinical research studies with ketamine].Encephale. 2001 Jan-Feb;27(1):53-9. Encephale. 2001. PMID: 11294039 Review. French.
-
Potential involvement of serotonergic signaling in ketamine's antidepressant actions: A critical review.Prog Neuropsychopharmacol Biol Psychiatry. 2016 Nov 3;71:27-38. doi: 10.1016/j.pnpbp.2016.05.007. Epub 2016 Jun 2. Prog Neuropsychopharmacol Biol Psychiatry. 2016. PMID: 27262695 Review.
References
-
- Wong D, Atiya S, Fogarty J, et al. Reduced hippocampal glutamate and posterior cingulate N-acetyl aspartate in mild cognitive impairment and Alzheimer’s disease is associated with episodic memory performance and white matter integrity in the cingulum: a pilot study. J Alzheimers Dis. 2020;73(4):1385 1405. (10.3233/JAD-190773) - DOI - PubMed
LinkOut - more resources
Full Text Sources
