SIRT1 improves lactate homeostasis in the brain to alleviate parkinsonism via deacetylation and inhibition of PKM2
- PMID: 39128469
- PMCID: PMC11384727
- DOI: 10.1016/j.xcrm.2024.101684
SIRT1 improves lactate homeostasis in the brain to alleviate parkinsonism via deacetylation and inhibition of PKM2
Abstract
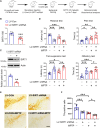
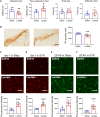
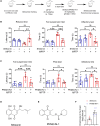
Sirtuin 1 (SIRT1) is a histone deacetylase and plays diverse functions in various physiological events, from development to lifespan regulation. Here, in Parkinson's disease (PD) model mice, we demonstrated that SIRT1 ameliorates parkinsonism, while SIRT1 knockdown further aggravates PD phenotypes. Mechanistically, SIRT1 interacts with and deacetylates pyruvate kinase M2 (PKM2) at K135 and K206, thus leading to reduced PKM2 enzyme activity and lactate production, which eventually results in decreased glial activation in the brain. Administration of lactate in the brain recapitulates PD-like phenotypes. Furthermore, increased expression of PKM2 worsens PD symptoms, and, on the contrary, inhibition of PKM2 by shikonin or PKM2-IN-1 alleviates parkinsonism in mice. Collectively, our data indicate that excessive lactate in the brain might be involved in the progression of PD. By improving lactate homeostasis, SIRT1, together with PKM2, are likely drug targets for developing agents for the treatment of neurodegeneration in PD.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The effect of PKM2-IN-1 in treating Parkinson’s disease has been patented (WO2022022748), and C.S. and Y.W. are listed as the inventors.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

