Novel furfural-complexed approach to synthesizing carbon-Doped ZnO with breakthrough photocatalytic efficacy
- PMID: 39128701
- PMCID: PMC12225955
- DOI: 10.1016/j.jare.2024.08.014
Novel furfural-complexed approach to synthesizing carbon-Doped ZnO with breakthrough photocatalytic efficacy
Abstract
Introduction: The efficiency of zinc oxide (ZnO) nanoparticles for environmental decontamination is limited by their reliance on ultraviolet (UV) light and rapid charge carrier recombination. Carbon doping has been proposed to address these challenges by potentially enhancing visible light absorption and charge separation.
Objectives: This study aims to introduce a novel, single-step synthesis method for carbon-doped ZnO (C-Z) nanoparticles, leveraging the decomposition of zinc nitrate hexahydrate and furfural under a nitrogen atmosphere to improve photocatalytic activity under visible light.
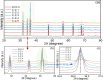
Methods: A series of C-Z variants (C-Z-1 to C-Z-5) and an undoped sample (ZnO-0) were synthesized. The influence of furfural on the synthesis process and doping mechanism was analyzed by X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), and UV-visible diffuse reflectance spectroscopy (DRS).
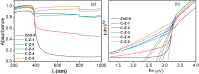
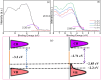
Results: XPS confirmed the integration of carbon within the ZnO matrix, and XRD indicated increased lattice dimensions owing to doping. DRS revealed bandgap narrowing, suggesting enhanced charge separation. Among the variants, C-Z-3 significantly outperformed the others, showing a 12-fold increase in the photocatalytic degradation rate of Rhodamine B compared to undoped ZnO.
Conclusion: The developed single-step synthesis method for C-Z nanoparticles represents a major advancement in materials engineering for ecological applications. The enhanced photocatalytic activity under visible light, as demonstrated by C-Z-3, underscores the potential of these nanoparticles for environmental decontamination.
Keywords: C-ZnO; Dye degradation; Furfural, Visible light; Photocatalysis.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Xue J., Ma S., Zhou Y., Zhang Z. Facile synthesis of ZnO-C nanocomposites with enhanced photocatalytic activity. New J Chem. 2015;39:1852–1857. doi: 10.1039/C4NJ02004A. - DOI
-
- Qiu S., Wu X., Xiao L., Ai X., Yang H., Cao Y. Antimony nanocrystals encapsulated in carbon microspheres synthesized by a facile self-catalyzing solvothermal method for high-performance sodium-ion battery anodes. ACS Appl Mater Interfaces. 2016;8:1337–1343. doi: 10.1021/acsami.5b10182. - DOI - PubMed
-
- Wainer P., Kendall O., Lamb A., Barrow S.J., Tricoli A., Gómez D.E., et al. Continuous growth synthesis of zinc oxide nanocrystals with tunable size and doping. Chem Mater. 2019;31:9604–9613. doi: 10.1021/acs.chemmater.9b02655. - DOI
LinkOut - more resources
Full Text Sources

