Potassium voltage-gated channel subfamily H member 2 (KCNH2) is a promising target for incretin secretagogue therapies
- PMID: 39128897
- PMCID: PMC11317495
- DOI: 10.1038/s41392-024-01923-z
Potassium voltage-gated channel subfamily H member 2 (KCNH2) is a promising target for incretin secretagogue therapies
Abstract
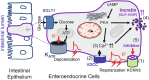
Derived from enteroendocrine cells (EECs), glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) are pivotal incretin hormones crucial for blood glucose regulation. Medications of GLP-1 analogs and GLP-1 receptor activators are extensively used in the treatment of type 2 diabetes (T2D) and obesity. However, there are currently no agents to stimulate endogenous incretin secretion. Here, we find the pivotal role of KCNH2 potassium channels in the regulation of incretin secretion. Co-localization of KCNH2 with incretin-secreting EECs in the intestinal epithelium of rodents highlights its significance. Gut epithelial cell-specific KCNH2 knockout in mice improves glucose tolerance and increases oral glucose-triggered GLP-1 and GIP secretion, particularly GIP. Furthermore, KCNH2-deficient primary intestinal epithelial cells exhibit heightened incretin, especially GIP secretion upon nutrient stimulation. Mechanistically, KCNH2 knockdown in EECs leads to reduced K+ currents, prolonged action potential duration, and elevated intracellular calcium levels. Finally, we found that dofetilide, a KCNH2-specific inhibitor, could promote incretin secretion in enteroendocrine STC-1 cells in vitro and in hyperglycemic mice in vivo. These findings elucidate, for the first time, the mechanism and application of KCNH2 in regulating incretin secretion by EECs. Given the therapeutic promise of GLP-1 and GIP in diabetes and obesity management, this study advances our understanding of incretin regulation, paving the way for potential incretin secretagogue therapies in the treatment of diabetes and obesity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

