MFGE8 promotes adult hippocampal neurogenesis in rats following experimental subarachnoid hemorrhage via modifying the integrin β3/Akt signaling pathway
- PMID: 39128910
- PMCID: PMC11317487
- DOI: 10.1038/s41420-024-02132-x
MFGE8 promotes adult hippocampal neurogenesis in rats following experimental subarachnoid hemorrhage via modifying the integrin β3/Akt signaling pathway
Abstract
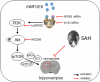
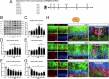
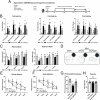
Subarachnoid hemorrhage (SAH) is one of the most severe type of cerebral strokes, which can cause multiple cellular changes in the brain leading to neuronal injury and neurological deficits. Specifically, SAH can impair adult neurogenesis in the hippocampal dentate gyrus, thus may affecting poststroke neurological and cognitive recovery. Here, we identified a non-canonical role of milk fat globule epidermal growth factor 8 (MFGE8) in rat brain after experimental SAH, involving a stimulation on adult hippocampal neurogenesis(AHN). Experimental SAH was induced in Sprague-Dawley rats via endovascular perforation, with the in vivo effect of MFGE8 evaluated via the application of recombinant human MFGE8 (rhMFGE8) along with pharmacological interventions, as determined by hemorrhagic grading, neurobehavioral test, and histological and biochemical analyses of neurogenesis related markers. Results: Levels of the endogenous hippocampal MFGE8 protein, integrin-β3 and protein kinase B (p-Akt) were elevated in the SAH relative to control groups, while that of hippocalcin (HPCA) and cyclin D1 showed the opposite change. Intraventricular rhMGFE8 infusion reversed the decrease in doublecortin (DCX) immature neurons in the DG after SAH, along with improved the short/long term neurobehavioral scores. rhMGFE8 treatment elevated the levels of phosphatidylinositol 3-kinase (PI3K), p-Akt, mammalian target of rapamycin (mTOR), CyclinD1, HPCA and DCX in hippocampal lysates, but not that of integrin β3 and Akt, at 24 hr after SAH. Treatment of integrin β3 siRNA, the PI3K selective inhibitor ly294002 or Akt selective inhibitor MK2206 abolished the effects of rhMGFE8 after SAH. In conclusion, MFGE8 is upregulated in the hippocampus in adult rats with reduced granule cell genesis. rhMFGE8 administration can rescue this impaired adult neurogenesis and improve neurobehavioral recovery. Mechanistically, the effect of MFGE8 on hippocampal adult neurogenesis is mediated by the activation of integrin β3/Akt pathway. These findings suggest that exogenous MFGE8 may be of potential therapeutic value in SAH management. Graphical abstract and proposed pathway of rhMFGE8 administration attenuate hippocampal injury by improving neurogenesis in SAH models. SAH caused hippocampal injury and neurogenesis interruption. Administered exogenous MFGE8, recombinant human MFGE8(rhMFGE8), could ameliorate hippocampal injury and improve neurological functions after SAH. Mechanistically, MFGE8 bind to the receptor integrin β3, which activated the PI3K/Akt pathway to increase the mTOR expression, and further promote the expression of cyclin D1, HPCA and DCX. rhMFGE8 could attenuated hippocampal injury by improving neurogenesis after SAH, however, know down integrin β3 or pharmacological inhibited PI3K/Akt by ly294002 or MK2206 reversed the neuro-protective effect of rhMFGE8.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
MFGE8/Integrin β3 pathway alleviates apoptosis and inflammation in early brain injury after subarachnoid hemorrhage in rats.Exp Neurol. 2015 Oct;272:120-7. doi: 10.1016/j.expneurol.2015.04.016. Epub 2015 May 1. Exp Neurol. 2015. PMID: 25936875 Free PMC article.
-
Milk Fat Globule-Epidermal Growth Factor-8 Pretreatment Attenuates Apoptosis and Inflammation via the Integrin-β3 Pathway after Surgical Brain Injury in Rats.Front Neurol. 2018 Feb 26;9:96. doi: 10.3389/fneur.2018.00096. eCollection 2018. Front Neurol. 2018. PMID: 29535679 Free PMC article.
-
Recombinant milk fat globule-EGF factor-8 reduces oxidative stress via integrin β3/nuclear factor erythroid 2-related factor 2/heme oxygenase pathway in subarachnoid hemorrhage rats.Stroke. 2014 Dec;45(12):3691-7. doi: 10.1161/STROKEAHA.114.006635. Epub 2014 Oct 23. Stroke. 2014. PMID: 25342030 Free PMC article.
-
Recombinant milk fat globule-EGF factor-8 reduces apoptosis via integrin β3/FAK/PI3K/AKT signaling pathway in rats after traumatic brain injury.Cell Death Dis. 2018 Aug 28;9(9):845. doi: 10.1038/s41419-018-0939-5. Cell Death Dis. 2018. PMID: 30154436 Free PMC article.
-
Recombinant OX40 attenuates neuronal apoptosis through OX40-OX40L/PI3K/AKT signaling pathway following subarachnoid hemorrhage in rats.Exp Neurol. 2020 Apr;326:113179. doi: 10.1016/j.expneurol.2020.113179. Epub 2020 Jan 10. Exp Neurol. 2020. PMID: 31930990 Review.
Cited by
-
Exomeres From Adventitial Fibroblasts of Spontaneously Hypertensive Rats Promote Vascular Remodelling via Transferring Osteopontin.J Extracell Vesicles. 2025 Aug;14(8):e70146. doi: 10.1002/jev2.70146. J Extracell Vesicles. 2025. PMID: 40767027 Free PMC article.
-
Hypoimmunogenic Human iPSCs for Repair and Regeneration in the CNS.Cells. 2025 Aug 13;14(16):1248. doi: 10.3390/cells14161248. Cells. 2025. PMID: 40862727 Free PMC article. Review.
-
Mossy fiber expression of αSMA in human hippocampus and its relevance to brain evolution and neuronal development.Sci Rep. 2025 May 6;15(1):15834. doi: 10.1038/s41598-025-00094-3. Sci Rep. 2025. PMID: 40328887 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

