Platelet extracellular vesicles preserve lymphatic endothelial cell integrity and enhance lymphatic vessel function
- PMID: 39128945
- PMCID: PMC11317532
- DOI: 10.1038/s42003-024-06675-8
Platelet extracellular vesicles preserve lymphatic endothelial cell integrity and enhance lymphatic vessel function
Abstract
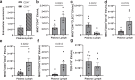
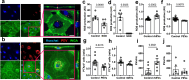
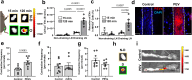
Lymphatic vessels are essential for preventing the accumulation of harmful components within peripheral tissues, including the artery wall. Various endogenous mechanisms maintain adequate lymphatic function throughout life, with platelets being essential for preserving lymphatic vessel integrity. However, since lymph lacks platelets, their impact on the lymphatic system has long been viewed as restricted to areas where lymphatics intersect with blood vessels. Nevertheless, platelets can also exert long range effects through the release of extracellular vesicles (EVs) upon activation. We observed that platelet EVs (PEVs) are present in lymph, a compartment to which they could transfer regulatory effects of platelets. Here, we report that PEVs in lymph exhibit a distinct signature enabling them to interact with lymphatic endothelial cells (LECs). In vitro experiments show that the internalization of PEVs by LECs maintains their functional integrity. Treatment with PEVs improves lymphatic contraction capacity in atherosclerosis-prone mice. We suggest that boosting lymphatic pumping with exogenous PEVs offers a novel therapeutic approach for chronic inflammatory diseases characterized by defective lymphatics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

