The dopamine analogue CA140 alleviates AD pathology, neuroinflammation, and rescues synaptic/cognitive functions by modulating DRD1 signaling or directly binding to Abeta
- PMID: 39129007
- PMCID: PMC11317008
- DOI: 10.1186/s12974-024-03180-x
The dopamine analogue CA140 alleviates AD pathology, neuroinflammation, and rescues synaptic/cognitive functions by modulating DRD1 signaling or directly binding to Abeta
Abstract
Background: We recently reported that the dopamine (DA) analogue CA140 modulates neuroinflammatory responses in lipopolysaccharide-injected wild-type (WT) mice and in 3-month-old 5xFAD mice, a model of Alzheimer's disease (AD). However, the effects of CA140 on Aβ/tau pathology and synaptic/cognitive function and its molecular mechanisms of action are unknown.
Methods: To investigate the effects of CA140 on cognitive and synaptic function and AD pathology, 3-month-old WT mice or 8-month-old (aged) 5xFAD mice were injected with vehicle (10% DMSO) or CA140 (30 mg/kg, i.p.) daily for 10, 14, or 17 days. Behavioral tests, ELISA, electrophysiology, RNA sequencing, real-time PCR, Golgi staining, immunofluorescence staining, and western blotting were conducted.
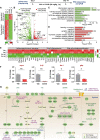
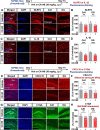
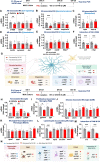
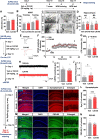
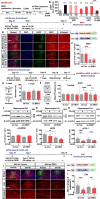
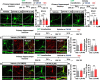
Results: In aged 5xFAD mice, a model of AD pathology, CA140 treatment significantly reduced Aβ/tau fibrillation, Aβ plaque number, tau hyperphosphorylation, and neuroinflammation by inhibiting NLRP3 activation. In addition, CA140 treatment downregulated the expression of cxcl10, a marker of AD-associated reactive astrocytes (RAs), and c1qa, a marker of the interaction of RAs with disease-associated microglia (DAMs) in 5xFAD mice. CA140 treatment also suppressed the mRNA levels of s100β and cxcl10, markers of AD-associated RAs, in primary astrocytes from 5xFAD mice. In primary microglial cells from 5xFAD mice, CA140 treatment increased the mRNA levels of markers of homeostatic microglia (cx3cr1 and p2ry12) and decreased the mRNA levels of a marker of proliferative region-associated microglia (gpnmb) and a marker of lipid-droplet-accumulating microglia (cln3). Importantly, CA140 treatment rescued scopolamine (SCO)-mediated deficits in long-term memory, dendritic spine number, and LTP impairment. In aged 5xFAD mice, these effects of CA140 treatment on cognitive/synaptic function and AD pathology were regulated by dopamine D1 receptor (DRD1)/Elk1 signaling. In primary hippocampal neurons and WT mice, CA140 treatment promoted long-term memory and dendritic spine formation via effects on DRD1/CaMKIIα and/or ERK signaling.
Conclusions: Our results indicate that CA140 improves neuronal/synaptic/cognitive function and ameliorates Aβ/tau pathology and neuroinflammation by modulating DRD1 signaling in primary hippocampal neurons, primary astrocytes/microglia, WT mice, and aged 5xFAD mice.
Keywords: Aβ; CA140; Dopamine D1 receptor; LTP; Learning and memory; Reactive gliosis; Tau.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27(5):457–64. - PubMed
MeSH terms
Substances
Grants and funding
- 2022R1A2C1011793/the National Research Foundation of Korea (NRF)
- 2021R1A4A1031644, 2021M3A9G8022960, 2022M325E8017907/the National Research Foundation of Korea (NRF)
- R01 AG053577/AG/NIA NIH HHS/United States
- R01AG053577/National Institute on Aging at the National Institutes of Health
- RS-2023-00253215/NRF
- 23-BR-01-02/Korea Brain Research Institute
- C320000/Korea Basic Science Institute
- 2019R1A2B5B01070108/the National Research Foundation of Korea (NRF)
- 24-BR-02-03, 23-BR-02-12, 24-BR-03-07, 24-BR-05-02, 24-BR-03-01, and 24-BR-03-05/Korea Brain Research Institute
- 21-BR-02-12/Korea Brain Research Institute
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

