PITX2 deficiency leads to atrial mitochondrial dysfunction
- PMID: 39129206
- PMCID: PMC11630043
- DOI: 10.1093/cvr/cvae169
PITX2 deficiency leads to atrial mitochondrial dysfunction
Abstract
Aims: Reduced left atrial PITX2 is associated with atrial cardiomyopathy and atrial fibrillation (AF). PITX2 is restricted to left atrial cardiomyocytes (aCMs) in the adult heart. The links between PITX2 deficiency, atrial cardiomyopathy, and AF are not fully understood.
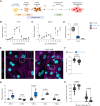
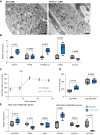
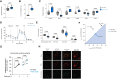
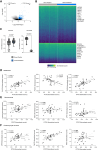
Methods and results: To identify mechanisms linking PITX2 deficiency to AF, we generated and characterized PITX2-deficient human aCMs derived from human induced pluripotent stem cells (hiPSC) and their controls. PITX2-deficient hiPSC-derived atrial cardiomyocytes showed shorter and disorganized sarcomeres and increased mononucleation. Electron microscopy found an increased number of smaller mitochondria compared with isogenic controls. Mitochondrial protein expression was altered in PITX2-deficient hiPSC-derived atrial cardiomyocytes. Single-nuclear RNA-sequencing found differences in cellular respiration pathways and differentially expressed mitochondrial and ion channel genes in PITX2-deficient hiPSC-derived atrial cardiomyocytes. PITX2 repression in hiPSC-derived atrial cardiomyocytes replicated dysregulation of cellular respiration. Mitochondrial respiration was shifted to increased glycolysis in PITX2-deficient hiPSC-derived atrial cardiomyocytes. PITX2-deficient human hiPSC-derived atrial cardiomyocytes showed higher spontaneous beating rates. Action potential duration was more variable with an overall prolongation of early repolarization, consistent with metabolic defects. Gene expression analyses confirmed changes in mitochondrial genes in left atria from 42 patients with AF compared with 43 patients with sinus rhythm. Dysregulation of left atrial mitochondrial (COX7C) and metabolic (FOXO1) genes was associated with PITX2 expression in human left atria.
Conclusion: PITX2 deficiency causes atrial mitochondrial dysfunction and a metabolic shift to glycolysis in human aCMs. PITX2-dependent metabolic changes can contribute to the structural and functional defects found in PITX2-deficient atria.
Keywords: PITX2; Atrial fibrillation; Human heart tissue; Human induced pluripotent stem cells; Metabolic shift; Mitochondrial dysfunction.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: L.F. has received institutional research grants and non-financial support from European Union, British Heart Foundation, Medical Research Council (U.K.), DFG, German Centre for Heart Research DZHK and several biomedical companies. P.K. has received additional support for research from the European Union, British Heart Foundation, Foundation Leducq, Medical Research Council (U.K.), and German Centre for Cardiovascular Research, from several drug and device companies active in AF, Honoria from several such companies, but not in the last three years. P.K. and L.F. are listed as inventors on two patents held by University of Birmingham (AFTherapy WO 2015140571, Markers for AF WO 2016021783). U.S. has received consultancy fees or honoraria from Università della Svizzera Italiana (USI, Switzerland), Roche Diagnostics (Switzerland), EP Solutions Inc. (Switzerland), Johnson & Johnson Medical Limited, (U.K.), Bayer Healthcare (Germany). U.S. is co-founder and shareholder of YourRhythmics BV, a spin-off company of the University Maastricht. K.G. has received additional support for research from the British Heart Foundation, Medical Research Council (U.K.), and Rocket Pharmaceuticals Inc. All other authors declare they have no competing interests.
Figures




References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. - PubMed
-
- Kirchhof P. The future of atrial fibrillation management: integrated care and stratified therapy. Lancet 2017;390:1873–1887. - PubMed
-
- Linz D, Andrade JG, Arbelo E, Boriani G, Breithardt G, Camm AJ, Caso V, Nielsen JC, De Melis M, De Potter T, Dichtl W, Diederichsen SZ, Dobrev D, Doll N, Duncker D, Dworatzek E, Eckardt L, Eisert C, Fabritz L, Farkowski M, Filgueiras-Rama D, Goette A, Guasch E, Hack G, Hatem S, Haeusler KG, Healey JS, Heidbuechel H, Hijazi Z, Hofmeister LH, Hove-Madsen L, Huebner T, Kaab S, Kotecha D, Malaczynska-Rajpold K, Merino JL, Metzner A, Mont L, Ng GA, Oeff M, Parwani AS, Puererfellner H, Ravens U, Rienstra M, Sanders P, Scherr D, Schnabel R, Schotten U, Sohns C, Steinbeck G, Steven D, Toennis T, Tzeis S, van Gelder IC, van Leerdam RH, Vernooy K, Wadhwa M, Wakili R, Willems S, Witt H, Zeemering S, Kirchhof P. Longer and better lives for patients with atrial fibrillation: the 9th AFNET/EHRA consensus conference. Europace 2024;26:euae070. - PMC - PubMed
-
- Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckley CM, Camm AJ, Casadei B, Chua W, Dagres N, de Melis M, Desteghe L, Diederichsen SZ, Duncker D, Eckardt L, Eisert C, Engler D, Fabritz L, Freedman B, Gillet L, Goette A, Guasch E, Svendsen JH, Hatem SN, Haeusler KG, Healey JS, Heidbuchel H, Hindricks G, Hobbs FDR, Hubner T, Kotecha D, Krekler M, Leclercq C, Lewalter T, Lin H, Linz D, Lip GYH, Lochen ML, Lucassen W, Malaczynska-Rajpold K, Massberg S, Merino JL, Meyer R, Mont L, Myers MC, Neubeck L, Niiranen T, Oeff M, Oldgren J, Potpara TS, Psaroudakis G, Purerfellner H, Ravens U, Rienstra M, Rivard L, Scherr D, Schotten U, Shah D, Sinner MF, Smolnik R, Steinbeck G, Steven D, Svennberg E, Thomas D, True Hills M, van Gelder IC, Vardar B, Pala E, Wakili R, Wegscheider K, Wieloch M, Willems S, Witt H, Ziegler A, Daniel Zink M, Kirchhof P. Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace 2023;25:6–27. - PMC - PubMed
-
- Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RC, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 2007;448:353–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- German Centre for Cardiovascular Research
- German Ministry of Education and Research
- NC/T001747/1/National Centre for the Replacement Refinement & Reduction of Animals in Research
- 509167694/Deutsche Forschungsgemeinschaft
- CVON2014-09/Netherlands Heart Foundation
- AA/18/2/34218/British Heart Foundation Accelerator Award
- Chan Zuckerberg Foundation
- AdG788970/European Union Horizon 2020 Research and Innovation Program
- FS/IBSRF/20/25039/British Heart Foundation Intermediate Fellow
- UK Research and Innovation
- HDRUK/CFC/01/MRC Health Data Research UK
- FS/13/43/30324/British Heart Foundation/United Kingdom
- 04510012110049/ZonMW Off-Road research
- 140VD01/Leducq Foundation
- 633196/European Commission
- 03-004-2022-0036/Dutch Heart Foundation
- 218649/Z/19/Z/Sir Henry Wellcome Fellows
- Department of Health and Social Care
- MR/V009540/1/Medical Research Council/United Kingdom
- Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

