Association of cortical morphology, white matter hyperintensity, and glymphatic function in frontotemporal dementia variants
- PMID: 39129270
- PMCID: PMC11497707
- DOI: 10.1002/alz.14158
Association of cortical morphology, white matter hyperintensity, and glymphatic function in frontotemporal dementia variants
Abstract
Introduction: Frontotemporal dementia (FTD) can be phenotypically divided into behavioral variant FTD (bvFTD), nonfluent variant primary progressive aphasia (nfvPPA), and semantic variant PPA (svPPA). However, the neural underpinnings of this phenotypic heterogeneity remain elusive.
Methods: Cortical morphology, white matter hyperintensities (WMH), diffusion tensor image analysis along the perivascular space (DTI-ALPS), and their interrelationships were assessed in subtypes of FTD. Neuroimaging-transcriptional analyses on the regional cortical morphological deviances among subtypes were also performed.
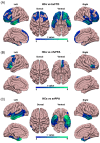
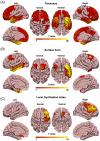
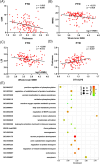
Results: Changes in cortical thickness, surface area, gyrification, WMH, and DTI-ALPS were subtype-specific in FTD. The three morphologic indices are related to whole-brain WMH volume and cognitive performance, while cortical thickness is related to DTI-ALPS. Neuroimaging-transcriptional analyses identified key biological pathways linked to the formation and/or spread of TDP-43/tau pathologies.
Discussion: We found subtype-specific changes in cortical morphology, WMH, and glymphatic function in FTD. Our findings have the potential to contribute to the development of personalized predictions and treatment strategies for this disorder.
Highlights: Cortical morphologic changes, white matter hyperintensities (WMH), and glymphatic dysfunction are subtype-specific. Cortical morphologic changes, WMH, and glymphatic dysfunction are inter-correlated. Cortical morphologic changes and WMH burden contribute to cognitive impairments.
Keywords: MRI; cortical morphology; frontotemporal dementia; glymphatic function; neuroimaging‐transcriptional association; white matter hyperintensities.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Authors declare that they have no competing interests. Author disclosures are available in the supporting information.
Figures


References
Publication types
MeSH terms
Grants and funding
- 82001784/National Natural Science Foundation of China
- 2021JJ41054/Natural Science Foundation (Youth Science Foundation Project) of Hunan Province
- 2019Q16/Youth Science Foundation of Xiangya Hospital
- 2022ZDZX2086/Key Platform Project of Department of Education of Guangdong Province
- Y03023206100209/Incubation Program for Innovative Science and Technology in UESTC
LinkOut - more resources
Full Text Sources

