Mineralocorticoid Receptor Antagonism Prevents Aortic Plaque Progression and Reduces Left Ventricular Mass and Fibrosis in Patients With Type 2 Diabetes and Chronic Kidney Disease: The MAGMA Trial
- PMID: 39129649
- PMCID: PMC11503525
- DOI: 10.1161/CIRCULATIONAHA.123.067620
Mineralocorticoid Receptor Antagonism Prevents Aortic Plaque Progression and Reduces Left Ventricular Mass and Fibrosis in Patients With Type 2 Diabetes and Chronic Kidney Disease: The MAGMA Trial
Abstract
Background: Persistent mineralocorticoid receptor activation is a pathologic response in type 2 diabetes and chronic kidney disease. Whereas mineralocorticoid receptor antagonists are beneficial in reducing cardiovascular complications, direct mechanistic pathways for these effects in humans are lacking.
Methods: The MAGMA trial (Mineralocorticoid Receptor Antagonism Clinical Evaluation in Atherosclerosis) was a randomized, double-blind, placebo-controlled trial in patients with high-risk type 2 diabetes with chronic kidney disease (not receiving dialysis) on maximum tolerated renin-angiotensin system blockade. The primary end point was change in thoracic aortic wall volume, expressed as absolute or percent value (ΔTWV or ΔPWV), using 3T magnetic resonance imaging at 12 months. Secondary end points were changes in left ventricle (LV) mass; LV fibrosis, measured as a change in myocardial native T1; and 24-hour ambulatory and central aortic blood pressures. Tertiary end points included plasma proteomic changes in 7596 plasma proteins using an aptamer-based assay.
Results: A total of 79 patients were randomized to placebo (n=42) or 25 mg of spironolactone daily (n=37). After a modified intent-to-treat, including available baseline data of study end points, patients who completed the trial protocol were included in the final analyses. At the 12-month follow-up, the average change in PWV was 7.1±10.7% in the placebo group and 0.87±10.0% in the spironolactone group (P=0.028), and ΔTWV was 1.2±1.7 cm3 in the placebo group and 0.037±1.9 cm3 in the spironolactone group (P=0.022). Change in LV mass was 3.1±8.4 g in the placebo group and -5.8±8.4 g in the spironolactone group (P=0.001). Changes in LV T1 values were significantly different between the placebo and spironolactone groups (26.0±41.9 ms in the placebo group versus a decrease of -10.1±36.3 ms in the spironolactone group; P=6.33×10-4). Mediation analysis revealed that the spironolactone effect on thoracic aortic wall volume and myocardial mass remained significant after adjustment for ambulatory and central blood pressures. Proteomic analysis revealed a dominant effect of spironolactone on pathways involving oxidative stress, inflammation, and leukocyte activation.
Conclusions: Among patients with diabetes with moderate to severe chronic kidney disease at elevated cardiovascular risk, treatment with spironolactone prevented progression of aortic wall volume and resulted in regression of LV mass and favorable alterations in native T1, suggesting amelioration of left-ventricular fibrosis.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT02169089.
Keywords: atherosclerosis; diabetes mellitus, type 2; mineralocorticoid receptor antagonists; renal insufficiency, chronic.
Conflict of interest statement
Dr Connelly holds the Keenan Chair in Research Leadership at St Michael’s Hospital and University of Toronto.
Figures


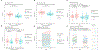



References
-
- Association AD. ADA Evidence Grading System for Clinical Practice Recommendations. Diabetes Care. 2008;31. doi: 10.2337/dc08 - DOI
-
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

