Adrenodoxin allosterically alters human cytochrome P450 11B enzymes to accelerate substrate binding and decelerate release
- PMID: 39129792
- PMCID: PMC11310744
- DOI: 10.1039/d4cb00015c
Adrenodoxin allosterically alters human cytochrome P450 11B enzymes to accelerate substrate binding and decelerate release
Abstract
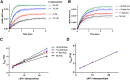
Two human mitochondrial membrane CYP11B enzymes play a pivotal role in steroidogenesis. CYP11B1 generates the major glucocorticoid cortisol, while CYP11B2 catalysis yields the primary mineralocorticoid aldosterone. Catalysis by both requires electron delivery by a soluble iron-sulfur adrenodoxin redox partner. However recent studies have shown that adrenodoxin/CYP11B interaction alone allosterically increases substrate and inhibitor affinity as exhibited by decreased dissociation constant (K d) values. The current study moves beyond such equilibrium studies, by defining adrenodoxin effects on the rates of P450 ligand binding and release separately. Stopped-flow data clearly demonstrate that adrenodoxin interaction with the P450 proximal surfaces increases ligand binding in both P450 CYP11B active sites by increasing the on rate constant and decreasing the off rate constant. As substrate entry and exit from the sequestered P450 active site requires conformational changes on the distal side of the P450 enzyme, a likely explanation is that adrenodoxin binding allosterically modulates CYP11B conformational changes. The 93% identical CYP11B enzymes can bind and hydroxylate each other's native substrates differing only by a hydroxyl. However, CYP11B1 exhibits monophasic substrate binding and CYP11B2 biphasic substrate binding, even when the substrates are swapped. This indicates that small differences in amino acid sequence between human CYP11B1 and CYP11B2 enzymes are more functionally important in ligand binding and could suggest avenues for more selective inhibition of these drug targets. Both protein/protein interactions and protein/substrate interactions are most likely to act by modulating CYP11B conformational dynamics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

