Chem4Energy: a consortium of the Royal Society Africa Capacity-Building Initiative
- PMID: 39129852
- PMCID: PMC11310705
- DOI: 10.1098/rsfs.2024.0001
Chem4Energy: a consortium of the Royal Society Africa Capacity-Building Initiative
Abstract
The Africa Capacity-Building Initiative is a Royal Society programme funded by the former UK Department for International Development to develop collaborative research between scientists in sub-Saharan Africa and the UK. Initially, four institutions were involved in the Chem4Energy consortium: Cardiff University in the UK and three African partners, the Kwame Nkrumah University of Science and Technology, Ghana, the University of Namibia and the University of Botswana, soon also including the Botswana International University of Science and Technology. The Chem4Energy research programme focused on 'New materials for a sustainable energy future: linking computation with experiment', aiming to deploy the synergy between state-of-the-art computational and experimental techniques to design and optimize new catalysts and semiconductor materials for renewable energy applications, based on materials that are abundant and readily available in African countries. The Chem4Energy consortium has achieved ambitious research goals, graduated seven PhD students and delivered a high-quality cross-disciplinary training programme in materials science and simulation techniques relevant to renewable energy applications. Since 2021, the extended consortium, including North-West University and the Centre for High-Performance Computing in South Africa, has remained active through an annual Chem4Energy conference series, with the sixth meeting taking place in Namibia in April 2025.
Keywords: capacity building; collaboration; sustainable energy.
© 2024 The Author(s).
Conflict of interest statement
We declare we have no competing interests.
Figures

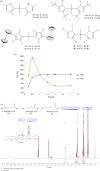
![Energy profile of the dehydrogenation of 1 using [Fe] and [Ru].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/9673/11310705/822a19023c66/rsfs.2024.0001.f004.gif)


References
-
- Frisch MJ, et al. . 2009. Gaussian 09, Revision B.01. Wallingford, CT: Gaussian, Inc.
LinkOut - more resources
Full Text Sources
Miscellaneous
