Obese Patients With Nonalcoholic Fatty Liver Disease Have an Increase in Soluble Plasma CD163 and a Concurrent Decrease in Hepatic Expression of CD163
- PMID: 39129874
- PMCID: PMC11307542
- DOI: 10.1016/j.gastha.2023.03.006
Obese Patients With Nonalcoholic Fatty Liver Disease Have an Increase in Soluble Plasma CD163 and a Concurrent Decrease in Hepatic Expression of CD163
Abstract
Background and aims: Macrophages play an important role in the development of nonalcoholic fatty liver disease (NAFLD) and its progression to nonalcoholic steatohepatitis (NASH). In this study, we investigated the hepatic expression of the macrophage scavenger receptor CD163 and the plasma level of its shed soluble form (sCD163) in patients with obesity and NASH, non-NASH NAFLD (NAFL), or healthy livers (no NAFLD).
Methods: Paired liver biopsies and plasma samples were collected from 61 patients with obesity (body mass index ≥35). Hepatic expression of CD163 was analyzed by immunohistochemistry and data-independent acquisition mass spectrometry, whilst plasma levels of sCD163 were determined by enzyme-linked immunosorbent assay and data-independent acquisition mass spectrometry. NAFLD stage and activity were assessed using the Kleiner fibrosis and NASH Clinical Research Network (NAS-CRN) scoring system.
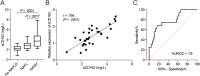
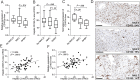
Results: sCD163 turned out as a promising predictor of NASH with an area under the receiver-operating characteristic curve of 0.78 [0.65;0.92] (P = .0008). sCD163 increased with more severe NAFLD both in univariate (odds ratio [OR] = 3.31[1.80;6.11], P < .001) and multivariable ordinal logistic regression adjusting for NAFLD risk factors (OR = 2.02 [1.03;3.97], P = .042). On the other hand, hepatic expression of CD163 was negatively associated with more severe NAFLD in univariate ordinal logistic regression determined by immunohistochemistry (OR = 0.91[0.84;0.98], P = .015) and proteomics (OR = 0.13[0.02;0.80], P = .028). Taking NAFLD risk factors into account, hepatic expression of CD163 was only associated with the fibrosis stage (OR = 0.01 [0.0003;0.21], P = .004). Accordingly, hepatic CD163 surface expression and sCD163 were negatively correlated (rho = -0.478, P = .0001).
Conclusion: An increased plasma sCD163 and a concurrent decreased hepatic expression of CD163 are strongly associated with NAFLD in obese patients.
Keywords: CD163; Immunohistochemistry; NAFLD; NASH; Proteomics; Translational.
© 2023 The Authors.
Figures



References
-
- Kazankov K., Jørgensen S.M.D., Thomsen K.L., et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16(3):145–159. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

