Impact of Stenting on PDA Length, Curvature, and Pulsatile Deformations Based on CT Assessment
- PMID: 39129905
- PMCID: PMC11307392
- DOI: 10.1016/j.jscai.2023.101134
Impact of Stenting on PDA Length, Curvature, and Pulsatile Deformations Based on CT Assessment
Abstract
Background: We sought to investigate the impact of stenting on native patent ductus arteriosus (PDA) length, curvature, and pulsatile deformations in patients with ductal-dependent pulmonary circulations.
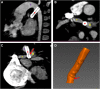
Methods: Patients with PDA stents who received contrast-enhanced 3-dimensional computed tomography with a view of the PDA, thoracic aorta, and pulmonary arteries were retrospectively included in this study. Geometric models of the prestented and poststented PDA were constructed from the computed tomography images, and PDA arclength, curvature, and pulsatile deformations were quantified.
Results: A total of 12 patients with cyanotic congenital heart disease were included, 10 of whom received 1 stent in the PDA and 2 received multiple overlapping stents. From prestenting to poststenting, the PDA shortened by 26 ± 18% (P = .004) and decreased in mean and peak curvature by 60 ± 21% and 68 ± 15%, respectively (both P < .001). Pulsatile deformations varied highly for the native PDA, stented PDA, and stents themselves.
Conclusions: The shortening and straightening of the PDA after stenting are significant and substantial, and their quantitative characterization will enable interventionalists to select stent lengths that span the entire PDA without encroaching on the aortic or pulmonary artery, which could cause hemodynamic interference, stent kink, and fatigue. Pulsatile PDA deformations can be used to design and evaluate devices tailored to congenital heart disease in neonates.
Keywords: device durability; device selection; ductal-dependent circulation; patent ductus arteriosus; preoperative planning; stenting.
© 2023 The Author(s).
Figures





Similar articles
-
Quantitative Analysis of 3D Anatomy to Inform Planning of Ductal Arteriosus Stenting.Catheter Cardiovasc Interv. 2025 Jun;105(7):1730-1739. doi: 10.1002/ccd.31510. Epub 2025 Mar 28. Catheter Cardiovasc Interv. 2025. PMID: 40152008 Free PMC article.
-
Correlation of ductus arteriosus length and morphology between computed tomographic angiography and catheter angiography and their relation to ductal stent length.Pediatr Radiol. 2020 May;50(6):800-809. doi: 10.1007/s00247-020-04624-1. Epub 2020 Mar 13. Pediatr Radiol. 2020. PMID: 32170350
-
Classification scheme for ductal morphology in cyanotic patients with ductal dependent pulmonary blood flow and association with outcomes of patent ductus arteriosus stenting.Catheter Cardiovasc Interv. 2019 Apr 1;93(5):933-943. doi: 10.1002/ccd.28125. Epub 2019 Feb 21. Catheter Cardiovasc Interv. 2019. PMID: 30790426
-
Stenting the complex patent ductus arteriosus in tetralogy of Fallot with pulmonary atresia: challenges and outcomes.Future Cardiol. 2018 Jan;14(1):55-73. doi: 10.2217/fca-2017-0053. Epub 2017 Dec 4. Future Cardiol. 2018. PMID: 29199861 Review.
-
PDA Stenting for Ductal-Dependent Cyanotic Congenital Heart Disease: History and View from 10,000 Feet.Pediatr Cardiol. 2024 Dec 17. doi: 10.1007/s00246-024-03737-w. Online ahead of print. Pediatr Cardiol. 2024. PMID: 39681751 Review.
Cited by
-
Quantitative Analysis of 3D Anatomy to Inform Planning of Ductal Arteriosus Stenting.Catheter Cardiovasc Interv. 2025 Jun;105(7):1730-1739. doi: 10.1002/ccd.31510. Epub 2025 Mar 28. Catheter Cardiovasc Interv. 2025. PMID: 40152008 Free PMC article.
-
Updates in interventional cardiology in children with cardiac disease.Eur J Pediatr. 2025 Jun 7;184(7):400. doi: 10.1007/s00431-025-06236-z. Eur J Pediatr. 2025. PMID: 40481951 Free PMC article. Review.
-
The Wire Twisting/Locking Technique to Facilitate Precise PDA Stent Delivery in Neonates with Ductal-Dependent Pulmonary Blood Flow.Pediatr Cardiol. 2025 May 5. doi: 10.1007/s00246-025-03852-2. Online ahead of print. Pediatr Cardiol. 2025. PMID: 40323405
References
-
- Mai C.T., Riehle-Colarusso T., O’halloran A., et al. Selected birth defects data from population-based birth defects surveillance programs in the United States, 2005-2009: featuring critical congenital heart defects targeted for pulse oximetry screening. Birth Defects Res A Clin Mol Teratol. 2012;94(12):970–983. doi: 10.1002/bdra.23098. - DOI - PMC - PubMed
-
- Mahle W.T., Newburger J.W., Matherne G.P., et al. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120(5):447–458. doi: 10.1161/CIRCULATIONAHA.109.192576. - DOI - PubMed

