Assessment of Noninferiority Margins in Cardiovascular Medicine Trials
- PMID: 39130003
- PMCID: PMC11312784
- DOI: 10.1016/j.jacadv.2024.101021
Assessment of Noninferiority Margins in Cardiovascular Medicine Trials
Abstract
Background: Noninferiority trials are increasingly common in cardiovascular medicine, but their reporting and interpretation are challenging, particularly when an absolute risk difference is used as noninferiority margin.
Objectives: This study aimed to investigate the effect of using absolute rather than relative noninferiority margins in cardiovascular trials.
Methods: We reviewed noninferiority trials presented at major cardiovascular conferences from 2015 to 2022 and published within the same period. Based on the actual versus anticipated event rates in the control group, we recalculated the absolute noninferiority margin and re-assessed the trial results. The primary outcome of interest was the proportion of trials with a different interpretation after recalculation. Additionally, we analyzed the conclusion statements of these trials to determine if cautionary notes for the interpretation of study results were included.
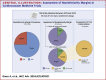
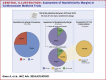
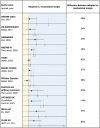
Results: We analyzed a total of 768 trials, of which 88 had a noninferiority design and 66 used an absolute noninferiority margin. Of 48 comparisons from 45 trials qualifying for the analysis, 11 (22.9%) had divergent results after recalculation of the absolute noninferiority margin based on the observed rather than anticipated event rate. Ten trials originally claiming noninferiority, did not meet it after the margin recalculation. All of them did not include statements suggesting cautionary interpretation of the study results in the conclusion section. Compared with the other trials, these displayed a larger median difference between anticipated and recalculated noninferiority margins (44.7% [IQR: 38.6%-56.7%] vs 15.3% [IQR: -1.5% to 28.9%]; P < 0.001).
Conclusions: Recalculating noninferiority margins based on actual event rates, rather than anticipated ones, led to different outcomes in approximately 1 out of 4 cardiovascular trials, with most divergent trials lacking cautionary interpretation. These findings emphasize the importance of using or supplementing the relative noninferiority margin, particularly in studies with significant deviations between observed and expected event rates. This underscores the critical need for enhanced methodological and reporting standards in noninferiority trials, especially those employing absolute margins.
Keywords: cardiovascular medicine; methodology; noninferiority; noninferiority margin; randomized trials; trial interpretation.
© 2024 The Authors.
Conflict of interest statement
Dr Capodanno has received honoraria from Novo Nordisk, Sanofi and Terumo, and Institutional fees from Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

Similar articles
-
Consequences of Inaccurate Assumptions in Coronary Stent Noninferiority Trials: A Systematic Review and Meta-analysis.JAMA Cardiol. 2022 Mar 1;7(3):320-327. doi: 10.1001/jamacardio.2021.5724. JAMA Cardiol. 2022. PMID: 35107583 Free PMC article.
-
Noninferiority Designed Cardiovascular Trials in Highest-Impact Journals.Circulation. 2019 Jul 30;140(5):379-389. doi: 10.1161/CIRCULATIONAHA.119.040214. Epub 2019 Jun 10. Circulation. 2019. PMID: 31177811 Free PMC article. Review.
-
Mixed noninferiority margin and statistical tests in active controlled trials.J Biopharm Stat. 2007;17(2):339-57. doi: 10.1080/10543400601183861. J Biopharm Stat. 2007. PMID: 17365228
-
A systematic review of noninferiority margins in oncology clinical trials.J Comp Eff Res. 2021 Apr;10(6):443-455. doi: 10.2217/cer-2020-0200. Epub 2021 Mar 17. J Comp Eff Res. 2021. PMID: 33728935
-
Comparison of noninferiority margins reported in protocols and publications showed incomplete and inconsistent reporting.J Clin Epidemiol. 2015 May;68(5):510-7. doi: 10.1016/j.jclinepi.2014.09.015. Epub 2014 Oct 22. J Clin Epidemiol. 2015. PMID: 25450451
Cited by
-
Stepwise dual antiplatelet therapy de-escalation in patients after drug coated balloon angioplasty (REC-CAGEFREE II): multicentre, randomised, open label, assessor blind, non-inferiority trial.BMJ. 2025 Mar 31;388:e082945. doi: 10.1136/bmj-2024-082945. BMJ. 2025. PMID: 40164448 Free PMC article. Clinical Trial.
-
Beta-blockers for secondary prevention following myocardial infarction in patients without reduced ejection fraction or heart failure: an updated meta-analysis.Eur J Prev Cardiol. 2025 Jun 3;32(8):633-646. doi: 10.1093/eurjpc/zwae298. Eur J Prev Cardiol. 2025. PMID: 39298680
-
Antiplatelet Monotherapies for Long-Term Secondary Prevention Following Percutaneous Coronary Intervention.J Clin Med. 2025 Aug 6;14(15):5536. doi: 10.3390/jcm14155536. J Clin Med. 2025. PMID: 40807157 Free PMC article. Review.
-
Characteristics and Impact of Randomized Trials on Drugs or Devices in Cardiovascular Medicine.Am J Cardiovasc Drugs. 2024 Sep;24(5):651-661. doi: 10.1007/s40256-024-00670-4. Epub 2024 Aug 1. Am J Cardiovasc Drugs. 2024. PMID: 39088111 Free PMC article.
References
-
- Collins R., MacMahon S. Reliable assessment of the effects of treatment on mortality and major morbidity, I: clinical trials. Lancet. 2001;357:373–380. - PubMed
-
- Zelen M. A new design for randomized clinical trials. N Engl J Med. 1979;300:1242–1245. - PubMed
-
- Ellenberg S.S. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 2: Practical issues and specific cases. Ann Intern Med. 2000;133:464. - PubMed
-
- Kates A.M., Morris P., Poppas A., Kuvin J.T. Impact of live, Scientific annual meetings in Today’s cardiovascular World. J Am Coll Cardiol. 2018;72:2082–2085. - PubMed
LinkOut - more resources
Full Text Sources

