Durvalumab-Induced Triple-M Syndrome
- PMID: 39130070
- PMCID: PMC11313117
- DOI: 10.12890/2024_004729
Durvalumab-Induced Triple-M Syndrome
Abstract
Background: While the use of immunotherapy has revolutionised the treatment of various cancers, it is often associated with a myriad of immune-related adverse effects.
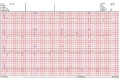
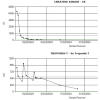
Case presentation: In this article, we report a rare case of durvalumab-induced triple-M syndrome in a 69-year-old woman with stage III lung adenocarcinoma. She was admitted with profound generalised muscle weakness, myalgia, and exertional breathlessness, about a week into her second cycle of durvalumab, an immune checkpoint inhibitor. She had clinicopathological features of myositis, myasthenia and myocarditis with acute onset symptomatic tri-fascicular block on electrocardiogram, requiring urgent cardiology intervention. Durvalumab was discontinued and she was treated with a combination of high-dose steroids and intravenous immunoglobulin after which she had clinical and biochemical improvement, albeit with residual muscle weakness.
Conclusion: Myocarditis-myositis-myasthenia complex is a rare side effect of immunotherapy which has been reported in other immune checkpoint inhibitors, but less so with durvalumab. We report this clinical case to raise awareness of this rare and potentially life-threatening adverse effect of this agent.
Learning points: Triple-M syndrome is a rare immune-related adverse effect, which has been noted in other immune checkpoint inhibitors, but less so with durvalumab specifically.Immunotherapy-induced myositis, myocarditis and myasthenia can occur in isolation or, rarely, in association as a syndrome.This case demonstrates the potentially life-threatening nature of this entity, the need for early recognition, and multi-specialist teamwork to ensure good outcome.
Keywords: Durvalumab; myastheniam; myocarditis; myositis; triple-M syndrome.
© EFIM 2024.
Conflict of interest statement
Conflicts of Interests: Dr. Islam reports receiving honoraria from AstraZeneca, Servier, Pfizer and BMS. All remaining authors have declared no conflicts of interest.
Figures


References
-
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. - PubMed
-
- Nosaki Y, Odate M, Takenaka H, Yokoi T, Iwai K. Development of myasthenia gravis: 4 years after durvalumab-induced myositis. Neurol Clin Neurosci. 2024;00:1–3.
LinkOut - more resources
Full Text Sources
