Adherence to Recommendations for Repeat Surveillance After Publication of New Postpolypectomy Guidelines
- PMID: 39130145
- PMCID: PMC11307611
- DOI: 10.1016/j.gastha.2022.07.014
Adherence to Recommendations for Repeat Surveillance After Publication of New Postpolypectomy Guidelines
Abstract
Background and aims: The 2012 and 2020 US Multi-Society Task Force postpolypectomy guidelines have recommended progressively longer surveillance intervals for patients with low-risk adenomas (LRAs). These guidelines require data from past colonoscopies. We examined the impact of the 2012 guidelines for second surveillance on clinical practice, including the availability of prior colonoscopy data, with the aim of informing the implementation of the 2020 guidelines.
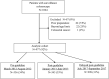
Methods: We identified surveillance colonoscopies at Stanford Health Care and the Palo Alto Veterans Affairs Health Care System in 3 periods: preguideline (March-August 2012), postguideline (January-June 2013), and delayed postguideline (July-September 2017). We collected data on the most recent previous colonoscopy, findings at the study entry surveillance colonoscopy, and recommendations for subsequent surveillance.
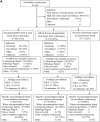
Results: Among 977 patients, the most recent prior colonoscopy data were available in 78% of preguideline, 78% of postguideline, and 61% of delayed postguideline cases (P < .001). The fraction of surveillance colonoscopy reports that deferred recommendations awaiting pathology increased from 6% to 11% in preguideline and postguideline to 59% in delayed postguideline cases (P < .001). Overall adherence to guidelines for subsequent surveillance was similar in all 3 periods (54%-67%; P = .089). In the postguideline and delayed postguideline periods combined, a 10-year subsequent surveillance interval was recommended in 0 of 29 cases with LRA followed by normal surveillance colonoscopy.
Conclusion: In patients undergoing surveillance, prior colonoscopy data were not always available and recommendations were often deferred awaiting pathology. Adherence to subsequent surveillance guidelines was suboptimal, especially for LRA followed by normal colonoscopy. Strategies addressing these gaps are needed to optimize implementation of the updated 2020 postpolypectomy guidelines.
Keywords: Colorectal Adenoma; Colorectal Polyps; Guidelines; Implementation; Surveillance.
© 2023 The Authors.
Figures


References
-
- Hardcastle J.D., Chamberlain J.O., Robinson M.H., et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. - PubMed
-
- Mandel J.S., Church T.R., Bond J.H., et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–1607. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

