Percutaneous Mechanical Aspiration in Infective Endocarditis: Applications, Technical Considerations, and Future Directions
- PMID: 39130180
- PMCID: PMC11307789
- DOI: 10.1016/j.jscai.2023.101269
Percutaneous Mechanical Aspiration in Infective Endocarditis: Applications, Technical Considerations, and Future Directions
Abstract
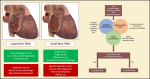
In recent years, there has been a shift in the epidemiology of patients with infective endocarditis (IE). This has been characterized by an alarming increase in IE in patients who inject drugs, cardiac implantable electronic device-related IE, and those with comorbid conditions and high surgical risk. This unmet need has mandated a reevaluation of complex management strategies in these patients and introduction of unconventional approaches in treatment. Percutaneous mechanical aspiration has emerged as both a diagnostic and therapeutic option in selected patients with IE. In this review, the authors discuss the gaps in care of IE, rationale, device armamentarium, procedural, and technical considerations and applications of percutaneous mechanical aspiration in IE.
Keywords: cardiovascular implantable electronic device; endocarditis; injection drug use; outcomes; percutaneous mechanical aspiration; tricuspid valve.
© 2023 The Author(s).
Conflict of interest statement
David Zlotnick is on the speaker’s bureau for Abiomed, Inari Medical. John Moriarty consults for AngioDynamics, Penumbra Medical, Innova Vascular, Pavmed, Pfizer, and Boston Scientific. Nadira Hamid consults for Abbott Structural, Anteris, AMX, 4C Medical Technologies, Alleviant Medical, Edwards Lifesciences, Philips, GE, Valcare Med, VDyne, and WL Gore. Yasir Akhtar received honoraria and is on speaker bureau for AngioDynamics and Penumbra. Kenneth Rosenfield consults for and/or is on the scientific advisory board member for Althea Medical, AngioDynamics, Boston Scientific, Contego, InspireMD, Magneto, Mayo Clinic, Neptune Medical, Philips, Summa Therapeutics, SurModics, Thrombolex, Terumo, and Truvic; holds equity in Accolade, Access Vascular, Aerami, Althea Medical, Contego, Cruzar Systems, Embolitech, Endospan, InspireMD, JanaCare, Magneto, Orchestra BioMed, PQ Bypass, Prosomnus, Shockwave Medical, Summa Therapeutics, Thrombolex, Truvic, and Valcare; and is a board member for National PERT Consortium. Christoph Starck received honoraria, consults for, and is on the advisory board of AngioDynamics, Abiomed, Atricure, Medtronic, Spectranetics, Biotronik, LivaNova (Sorin), and Cook Medical and received departmental or institutional research funding from Cook Medical and Hylomorph. Sripal Bangalore is on the advisory board for Abbott Vascular, Boston Scientific, Biotronik, Amgen, Pfizer, Merck, REATA, Inari Medical, and Truvic. Sahil Parikh receives institutional grants/research support from Abbott Vascular, Shockwave Medical, TriReme Medical, SurModics, Silk Road Medical, and the National Institutes of Health; has received consulting fees from Terumo and Abiomed; and has served on the advisory boards of Abbott, Medtronic, Boston Scientific, CSI, Janssen, and Philips. Sanjum Sethi reports honoraria from Janssen and Chiesi. Abdallah Sabbagh, Evin Yucel, Stephanie Younes, Larry Baddour, and Patrick O’Gara reported no financial interests.
Figures

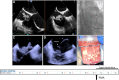
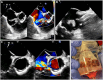
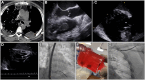
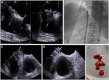





References
-
- Pant S., Patel N.J., Deshmukh A., et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65(19):2070–2076. - PubMed
-
- Cahill T.J., Prendergast B.D. Infective endocarditis. Lancet. 2016;387(10021):882–893. - PubMed

