Higher-order interactions and emergent properties of microbial communities: The power of synthetic ecology
- PMID: 39130413
- PMCID: PMC11315108
- DOI: 10.1016/j.heliyon.2024.e33896
Higher-order interactions and emergent properties of microbial communities: The power of synthetic ecology
Abstract
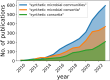
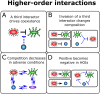
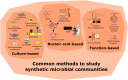
Humans have long relied on microbial communities to create products, produce energy, and treat waste. The microbiota residing within our bodies directly impacts our health, while the soil and rhizosphere microbiomes influence the productivity of our crops. However, the complexity and diversity of microbial communities make them challenging to study and difficult to develop into applications, as they often exhibit the emergence of unpredictable higher-order phenomena. Synthetic ecology aims at simplifying complexity by constituting synthetic or semi-natural microbial communities with reduced diversity that become easier to study and analyze. This strategy combines methodologies that simplify existing complex systems (top-down approach) or build the system from its constituent components (bottom-up approach). Simplified communities are studied to understand how interactions among populations shape the behavior of the community and to model and predict their response to external stimuli. By harnessing the potential of synthetic microbial communities through a multidisciplinary approach, we can advance knowledge of ecological concepts and address critical public health, agricultural, and environmental issues more effectively.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources

