HDAC6 inhibition as a mechanism to prevent neurodegeneration in the mSOD1G93A mouse model of ALS
- PMID: 39130445
- PMCID: PMC11315133
- DOI: 10.1016/j.heliyon.2024.e34587
HDAC6 inhibition as a mechanism to prevent neurodegeneration in the mSOD1G93A mouse model of ALS
Abstract
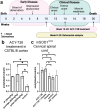
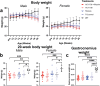
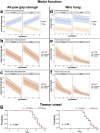
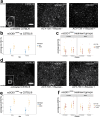
The loss of upper and lower motor neurons, and their axons is central to the loss of motor function and death in amyotrophic lateral sclerosis (ALS). Due to the diverse range of genetic and environmental factors that contribute to the pathogenesis of ALS, there have been difficulties in developing effective therapies for ALS. One emerging dichotomy is that protection of the neuronal cell soma does not prevent axonal vulnerability and degeneration, suggesting the need for targeted therapeutics to prevent axon degeneration. Post-translational modifications of protein acetylation can alter the function, stability and half-life of individual proteins, and can be enzymatically modified by histone acetyltransferases (HATs) and histone deacetyltransferases (HDACs), which add, or remove acetyl groups, respectively. Maintenance of post-translational microtubule acetylation has been suggested as a mechanism to stabilize axons, prevent axonal loss and neurodegeneration in ALS. This study used an orally dosed potent HDAC6 inhibitor, ACY-738, prevent deacetylation and stabilize microtubules in the mSOD1G93A mouse model of ALS. Co-treatment with riluzole was performed to determine any effects or drug interactions and potentially enhance preclinical research translation. This study shows ACY-738 treatment increased acetylation of microtubules in the spinal cord of mSOD1G93A mice, reduced lower motor neuron degeneration in female mice, ameliorated reduction in peripheral nerve axon puncta size, but did not prevent overt motor function decline. The current study also shows peripheral nerve axon puncta size to be partially restored after treatment with riluzole and highlights the importance of co-treatment to measure the potential effects of therapeutics in ALS.
Keywords: ACY-738; ALS; Axon degeneration; Neurofilament; Riluzole; SOD1.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

