Genetics and Therapeutic Responses to Tumor-Infiltrating Lymphocyte Therapy of Pancreatic Cancer Patient-Derived Xenograft Models
- PMID: 39131259
- PMCID: PMC11307969
- DOI: 10.1016/j.gastha.2022.07.006
Genetics and Therapeutic Responses to Tumor-Infiltrating Lymphocyte Therapy of Pancreatic Cancer Patient-Derived Xenograft Models
Abstract
Background and aims: Pancreatic cancer is the seventh leading cause of cancer-related deaths worldwide. Checkpoint immunotherapy has not yet shown encouraging results in pancreatic cancer possibly because of a poor immunogenicity and/or an immune suppressive microenvironment. The aim of this study was to develop patient-derived xenograft (PDX) models, compare their genetics to the original biopsies, and assess if autologous tumor-infiltrating lymphocytes (TILs) would have antitumoral activity in pancreatic cancer.
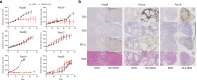
Methods: We subcutaneously transplanted tumors from 29 patients into NOG mice to generate PDX models. We established TIL cultures and injected them into PDX mice. We analyzed histology and genetics of biopsies and PDX tumors.
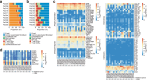
Results: Tumor growths were confirmed in 11 of 29 transplantations. The PDX tumors histologically resembled their original biopsies, but because stromal cells in the PDX model tumors were from mouse, their gene expression differed from the original biopsies. Immune checkpoint ligands other than programmed death ligand-1 (PD-L1) were expressed in pancreatic cancers, but PD-L1 was rarely expressed. When it was expressed, it correlated with tumor take in PDX models. One of the 3 tumors that expressed PD-L1 was an adenosquamous cancer, and another had a mismatch repair deficiency. TILs were expanded from 6 tumors and were injected into NOG or human interleukin-2 transgenic-NOG mice carrying PDX tumors. Regression of tumors could be verified in human interleukin-2 transgenic-NOG mice in 3 of the 6 PDX models treated with autologous TILs, including the adenosquamous PDX model.
Conclusion: PDX models of pancreatic cancer can be used to learn more about tumor characteristics and biomarkers and to evaluate responses to adoptive cell therapy and combination therapies. The major benefit of the model is that modifications of T cells can be tested in an autologous humanized mouse model to gain preclinical data to support the initiation of a clinical trial.
Keywords: Pancreatic Cancer; Patient-Derived Xenografts; Transcriptomic classification; Tumor-Infiltrating Lymphocytes.
© 2022 The Authors.
Figures


References
-
- Bray F., Ferlay J., Soerjomataram I., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. - PubMed
-
- Chen D.S., Irving B.A., Hodi F.S. Molecular pathways: next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580–6587. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

