This is a preprint.
Immunodominant extracellular loops of Treponema pallidum FadL outer membrane proteins elicit antibodies with opsonic and growth-inhibitory activities
- PMID: 39131275
- PMCID: PMC11312542
- DOI: 10.1101/2024.07.30.605823
Immunodominant extracellular loops of Treponema pallidum FadL outer membrane proteins elicit antibodies with opsonic and growth-inhibitory activities
Update in
-
Immunodominant extracellular loops of Treponema pallidum FadL outer membrane proteins elicit antibodies with opsonic and growth-inhibitory activities.PLoS Pathog. 2024 Dec 23;20(12):e1012443. doi: 10.1371/journal.ppat.1012443. eCollection 2024 Dec. PLoS Pathog. 2024. PMID: 39715273 Free PMC article.
Abstract
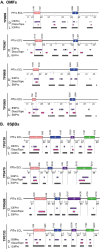
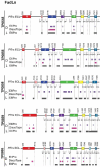
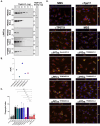
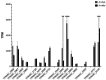
The global resurgence of syphilis has created a potent stimulus for vaccine development. To identify potentially protective antibodies (Abs) against Treponema pallidum (TPA), we used Pyrococcus furiosus thioredoxin (PfTrx) to display extracellular loops (ECLs) from three TPA outer membrane protein families (outer membrane factors for efflux pumps, eight-stranded β-barrels, and FadLs) to assess their reactivity with immune rabbit serum (IRS). Five ECLs from the FadL orthologs TP0856, TP0858 and TP0865 were immunodominant. Rabbits and mice immunized with these five PfTrx constructs produced ECL-specific Abs that promoted opsonophagocytosis of TPA by rabbit peritoneal and murine bone marrow-derived macrophages at levels comparable to IRS and mouse syphilitic serum. ECL-specific rabbit and mouse Abs also impaired viability, motility, and cellular attachment of spirochetes during in vitro cultivation. The results support the use of ECL-based vaccines and suggest that ECL-specific Abs promote spirochete clearance via Fc receptor-independent as well as Fc receptor-dependent mechanisms.
Keywords: PfTrx; Pyrococcus furiosus thioredoxin; Syphilis; Treponema pallidum; antibodies; extracellular loop; opsonophagocytosis; outer membrane protein; vaccine.
Conflict of interest statement
Competing Interests: All authors have no relevant financial or non-financial competing interests to report.
Figures






Similar articles
-
Immunodominant extracellular loops of Treponema pallidum FadL outer membrane proteins elicit antibodies with opsonic and growth-inhibitory activities.PLoS Pathog. 2024 Dec 23;20(12):e1012443. doi: 10.1371/journal.ppat.1012443. eCollection 2024 Dec. PLoS Pathog. 2024. PMID: 39715273 Free PMC article.
-
Extracellular Loops of the Treponema pallidum FadL Orthologs TP0856 and TP0858 Elicit IgG Antibodies and IgG+-Specific B-Cells in the Rabbit Model of Experimental Syphilis.mBio. 2022 Aug 30;13(4):e0163922. doi: 10.1128/mbio.01639-22. Epub 2022 Jul 12. mBio. 2022. PMID: 35862766 Free PMC article.
-
Use of Epivolve phage display to generate a monoclonal antibody with opsonic activity directed against a subdominant epitope on extracellular loop 4 of Treponema pallidum BamA (TP0326).Front Immunol. 2023 Aug 22;14:1222267. doi: 10.3389/fimmu.2023.1222267. eCollection 2023. Front Immunol. 2023. PMID: 37675118 Free PMC article.
-
Polypeptides of Treponema pallidum: progress toward understanding their structural, functional, and immunologic roles. Treponema Pallidum Polypeptide Research Group.Microbiol Rev. 1993 Sep;57(3):750-79. doi: 10.1128/mr.57.3.750-779.1993. Microbiol Rev. 1993. PMID: 8246847 Free PMC article. Review.
-
Notes on syphilis vaccine development.Front Immunol. 2022 Jul 28;13:952284. doi: 10.3389/fimmu.2022.952284. eCollection 2022. Front Immunol. 2022. PMID: 35967432 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
