This is a preprint.
Unraveling cysteine deficiency-associated rapid weight loss
- PMID: 39131293
- PMCID: PMC11312522
- DOI: 10.1101/2024.07.30.605703
Unraveling cysteine deficiency-associated rapid weight loss
Update in
-
Unravelling cysteine-deficiency-associated rapid weight loss.Nature. 2025 Jul;643(8072):776-784. doi: 10.1038/s41586-025-08996-y. Epub 2025 May 21. Nature. 2025. PMID: 40399674 Free PMC article.
Abstract
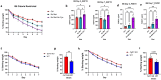
Forty percent of the US population and 1 in 6 individuals worldwide are obese, and the incidence of this disease is surging globally1,2. Various dietary interventions, including carbohydrate and fat restriction, and more recently amino acid restriction, have been explored to combat this epidemic3-6. We sought to investigate the impact of removing individual amino acids on the weight profiles of mice. Compared to essential amino acid restriction, induction of conditional cysteine restriction resulted in the most dramatic weight loss, amounting to 20% within 3 days and 30% within one week, which was readily reversed. This weight loss occurred despite the presence of substantial cysteine reserves stored in glutathione (GSH) across various tissues7. Further analysis demonstrated that the weight reduction primarily stemmed from an increase in the utilization of fat mass, while locomotion, circadian rhythm and histological appearance of multiple other tissues remained largely unaffected. Cysteine deficiency activated the integrated stress response (ISR) and NRF2-mediated oxidative stress response (OSR), which amplify each other, leading to the induction of GDF15 and FGF21, hormones associated with increased lipolysis, energy homeostasis and food aversion8-10. We additionally observed rapid tissue coenzyme A (CoA) depletion, resulting in energetically inefficient anaerobic glycolysis and TCA cycle, with sustained urinary excretion of pyruvate, orotate, citrate, α-ketoglutarate, nitrogen rich compounds and amino acids. In summary, our investigation highlights that cysteine restriction, by depleting GSH and CoA, exerts a maximal impact on weight loss, metabolism, and stress signaling compared to other amino acid restrictions. These findings may pave the way for innovative strategies for addressing a range of metabolic diseases and the growing obesity crisis.
Conflict of interest statement
Conflicts of Interest D.R.L consults for and has equity interest in Vedanta Bioscience, Sonoma Immunotherapeutics, Immunai, IMIDomics, and Pfizer, Inc.
Figures

















References
-
- Stierman B., A. J.; Carroll M. D.; Chen T. C.; Davy O.; Fink S.; Fryar C. D.; Gu Q.; Hales C. M.; Hughes J. P.; Ostchega Y.; Storandt R. J.; Akinbami L. J.. National health and nutrition examination survey 2017–march 2020 prepandemic data files-development of files and prevalence estimates for selected health outcomes. National Health Statistics Reports (2021). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
