This is a preprint.
Single-nanoparticle electrophoretic mobility determination and trapping using active-feedback 3D tracking
- PMID: 39131346
- PMCID: PMC11312477
- DOI: 10.1101/2024.07.08.602591
Single-nanoparticle electrophoretic mobility determination and trapping using active-feedback 3D tracking
Abstract
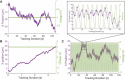
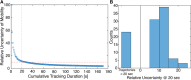
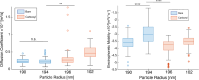
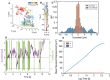
Nanoparticles (NP) are versatile materials with widespread applications across medicine and engineering. Despite rapid incorporation into drug delivery, therapeutics, and many more areas of research and development, there is a lack of robust characterization methods. Light scattering techniques such as dynamic light scattering (DLS) and electrophoretic light scattering (ELS) use an ensemble-averaged approach to the characterization of nanoparticle size and electrophoretic mobility (EM), leading to inaccuracies when applied to polydisperse or heterogeneous populations. To address this lack of single-nanoparticle characterization, this work applies 3D Single-Molecule Active Real-time Tracking (3D-SMART) to simultaneously determine NP size and EM on a per-particle basis. Single-nanoparticle EM is determined by using active feedback to "lock on" to a single particle and apply an oscillating electric field along one axis. A maximum likelihood approach is applied to extract the single-particle EM from the oscillating nanoparticle position along the field-actuated axis, while mean squared displacement is used along the non-actuated axes to determine size. Unfunctionalized and carboxyl-functionalized polystyrene NPs are found to have unique EM based on their individual size and surface characteristics, and it is demonstrated that single-nanoparticle EM is a more precise tool for distinguishing unique NP preparations than diffusion alone, able to determine the charge number of individual NPs to an uncertainty of less than 30. This method also explored individual nanoparticle EM in various ionic strengths (0.25-5 mM) and found decreased EM as a function of increasing ionic strength, in agreement with results determined via bulk characterization methods. Finally, it is demonstrated that the electric field can be manipulated in real time in response to particle position, resulting in one-dimensional electrokinetic trapping. Critically, this new single-nanoparticle EM determination and trapping method does not require microfluidics, opening the possibility for the exploration of single-nanoparticle EM in live tissue and more comprehensive characterization of nanoparticles in biologically relevant environments.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
