RAGE/DIAPH1 and atherosclerosis through an evolving lens: Viewing the cell from the "Inside - Out"
- PMID: 39131441
- PMCID: PMC11309734
- DOI: 10.1016/j.atherosclerosis.2023.117304
RAGE/DIAPH1 and atherosclerosis through an evolving lens: Viewing the cell from the "Inside - Out"
Abstract
Background and aims: In hyperglycemia, inflammation, oxidative stress and aging, Damage Associated Molecular Patterns (DAMPs) accumulate in conditions such as atherosclerosis. Binding of DAMPs to receptors such as the receptor for advanced glycation end products (RAGE) activates signal transduction cascades that contribute to cellular stress. The cytoplasmic domain (tail) of RAGE (ctRAGE) binds to the formin Diaphanous1 (DIAPH1), which is important for RAGE signaling. This Review will detail the evidence linking the RAGE/DIAPH1 signaling pathway to atherosclerosis and envisages future therapeutic opportunities from the "inside-out" point of view in affected cells.
Methods: PubMed was searched using a variety of search terms, including "receptor for advanced glycation end products" along with various combinations including "and atherosclerosis," "soluble RAGE and atherosclerosis," "statins and RAGE," "PPAR and RAGE" and "SGLT2 inhibitor and RAGE."
Results: In non-diabetic and diabetic mice, antagonism or global deletion of Ager (the gene encoding RAGE) retards progression and accelerates regression of atherosclerosis. Global deletion of Diaph1 in mice devoid of the low density lipoprotein receptor (Ldlr) significantly attenuates atherosclerosis; mice devoid of both Diaph1 and Ldlr display significantly lower plasma and liver concentrations of cholesterol and triglyceride compared to mice devoid of Ldlr. Associations between RAGE pathway and human atherosclerosis have been identified based on relationships between plasma/serum concentrations of RAGE ligands, soluble RAGEs and atherosclerosis.
Conclusions: Efforts to target RAGE/DIAPH1 signaling through a small molecule antagonist therapeutic strategy hold promise to quell accelerated atherosclerosis in diabetes and in other forms of cardiovascular disease.
Keywords: Atherosclerosis; Diabetes; Receptor for AGE; Soluble RAGEs.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: RR, AS and AMS have patents and patent applications through NYU Grossman School of Medicine that have been submitted/published that are related to some of the work reviewed in this manuscript.
Figures


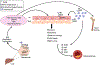
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

