Kinetics of Calcite Nucleation onto Sulfated Chitosan Derivatives and Implications for Water-Polysaccharide Interactions during Crystallization of Sparingly Soluble Salts
- PMID: 39131446
- PMCID: PMC11311137
- DOI: 10.1021/acs.cgd.4c00602
Kinetics of Calcite Nucleation onto Sulfated Chitosan Derivatives and Implications for Water-Polysaccharide Interactions during Crystallization of Sparingly Soluble Salts
Abstract
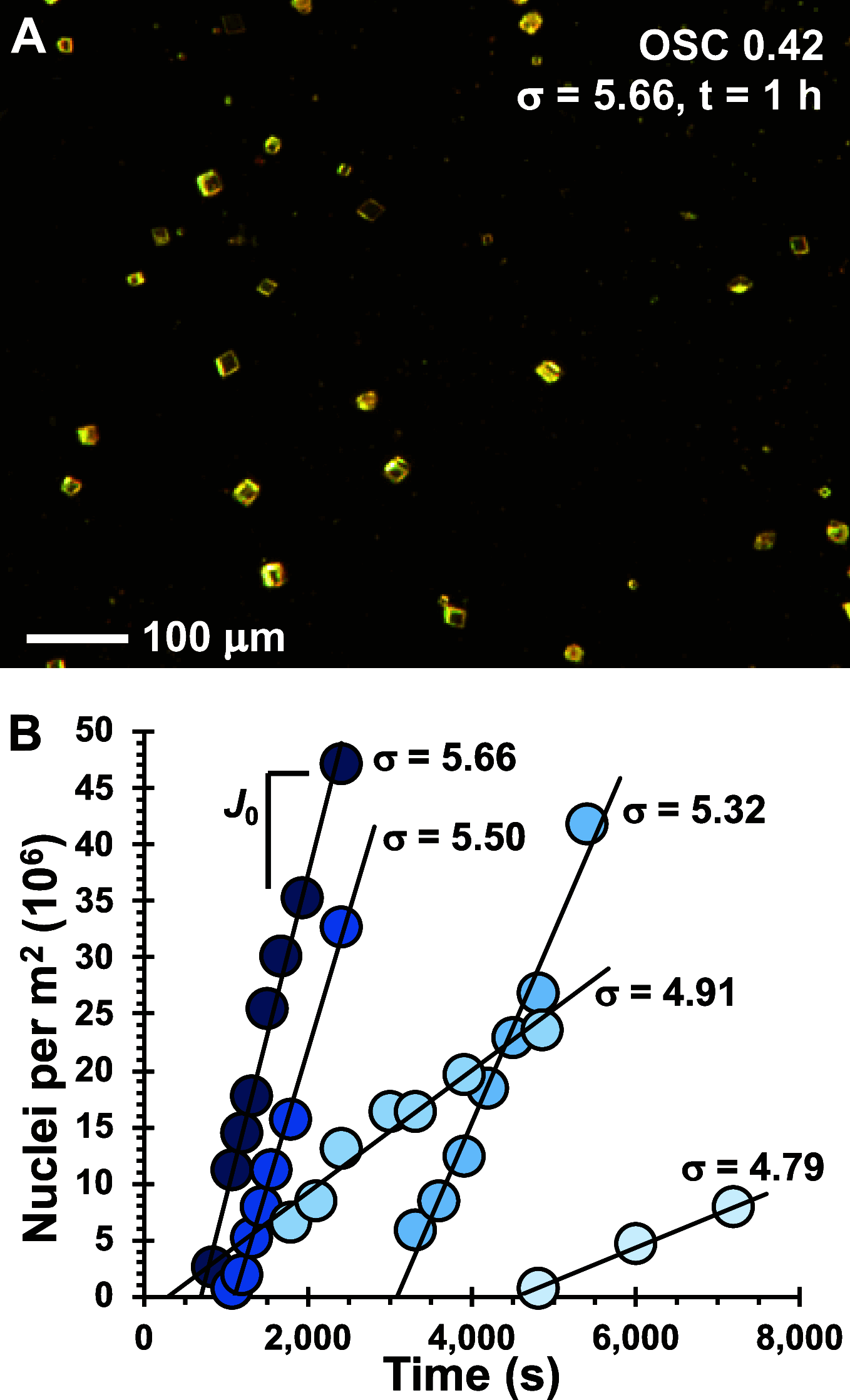
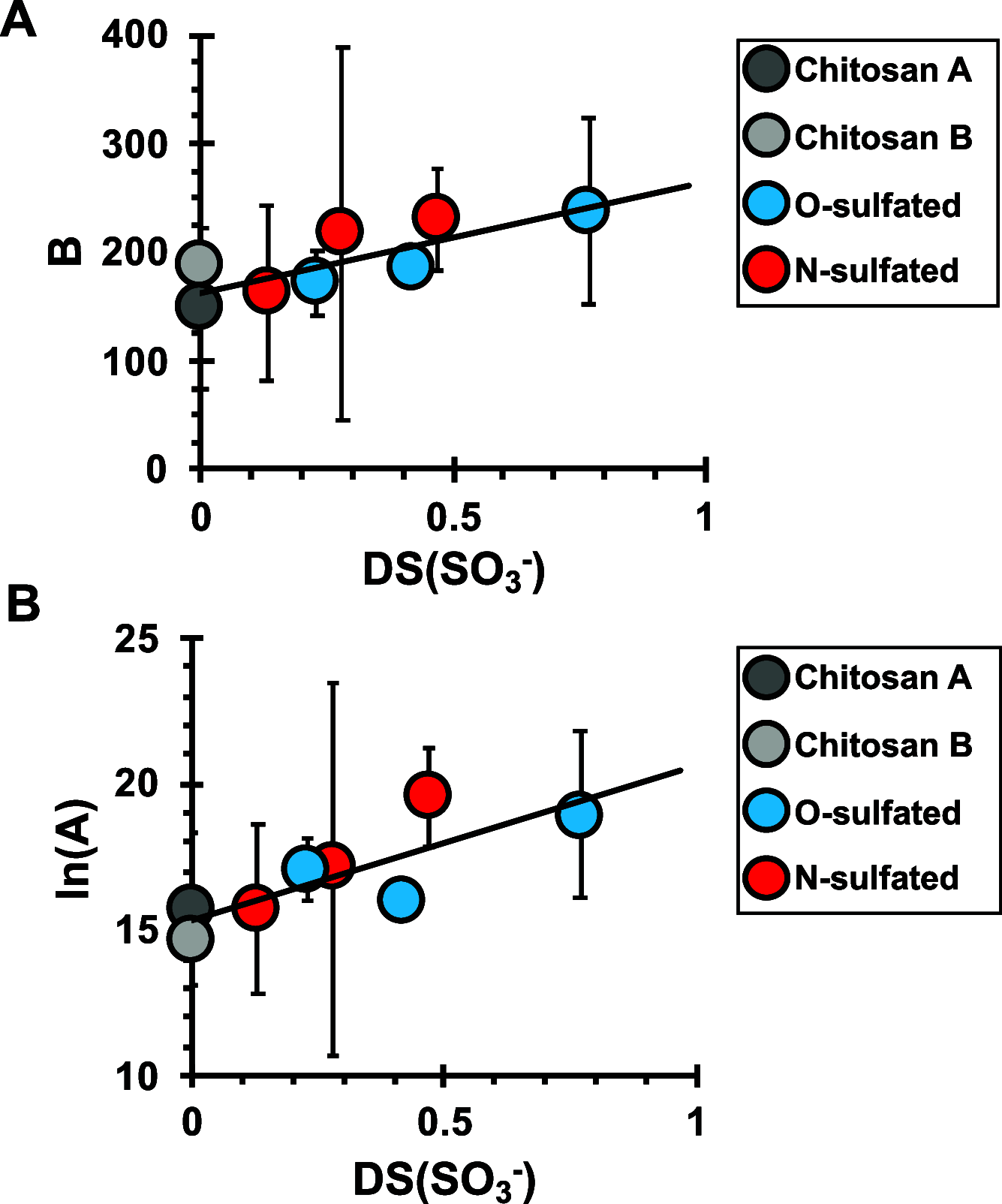
Anionic macromolecules are found at sites of CaCO3 biomineralization in diverse organisms, but their roles in crystallization are not well-understood. We prepared a series of sulfated chitosan derivatives with varied positions and degrees of sulfation, DS(SO3 -), and measured calcite nucleation rate onto these materials. Fitting the classical nucleation theory model to the kinetic data reveals the interfacial free energy of the calcite-polysaccharide-solution system, γnet, is lowest for nonsulfated controls and increases with DS(SO3 -). The kinetic prefactor also increases with DS(SO3 -). Simulations of Ca2+-H2O-chitosan systems show greater water structuring around sulfate groups compared to uncharged substituents, independent of sulfate location. Ca2+-SO3 - interactions are solvent-separated by distances that are inversely correlated with DS(SO3 -) of the polysaccharide. The simulations also predict SO3 - and NH3 + groups affect the solvation waters and HCO3 - ions associated with Ca2+. Integrating the experimental and computational evidence suggests sulfate groups influence nucleation by increasing the difficulty of displacing near-surface water, thereby increasing γnet. By correlating γnet and net charge per monosaccharide for diverse polysaccharides, we suggest the solvent-separated interactions of functional groups with Ca2+ influence thermodynamic and kinetic components to crystallization by similar solvent-dominated processes. The findings reiterate the importance of establishing water structure and properties at macromolecule-solution interfaces.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Polysaccharide chemistry regulates kinetics of calcite nucleation through competition of interfacial energies.Proc Natl Acad Sci U S A. 2013 Jun 4;110(23):9261-6. doi: 10.1073/pnas.1222162110. Epub 2013 May 20. Proc Natl Acad Sci U S A. 2013. PMID: 23690577 Free PMC article.
-
Chitosan as a Canvas for Studies of Macromolecular Controls on CaCO3 Biological Crystallization.Biomacromolecules. 2023 Mar 13;24(3):1078-1102. doi: 10.1021/acs.biomac.2c01394. Epub 2023 Feb 28. Biomacromolecules. 2023. PMID: 36853173 Review.
-
Thermodynamic and Kinetic Parameters for Calcite Nucleation on Peptoid and Model Scaffolds: A Step toward Nacre Mimicry.Cryst Growth Des. 2020 Jun 3;20(6):3762-3771. doi: 10.1021/acs.cgd.0c00029. Epub 2020 Apr 24. Cryst Growth Des. 2020. PMID: 33192182 Free PMC article.
-
Catalytic synthesis of sulfated polysaccharides I: Characterization of chemical structure.Int J Biol Macromol. 2015 Mar;74:61-7. doi: 10.1016/j.ijbiomac.2014.11.033. Epub 2014 Dec 8. Int J Biol Macromol. 2015. PMID: 25499892
-
Crystal Nucleation and Growth of Inorganic Ionic Materials from Aqueous Solution: Selected Recent Developments, and Implications.Small. 2022 Jul;18(28):e2107735. doi: 10.1002/smll.202107735. Epub 2022 Jun 9. Small. 2022. PMID: 35678091 Review.
References
-
- Nancollas G. H.Biological Mineralization; North-Holland, 1981.
-
- Lowenstam H. A.; Weiner S.. On Biomineralization; Oxford University Press, 1989.
-
- Simkiss K.; Wilbur K. M.. Biomineralization; Elsevier Science, 1989.
-
- Wheeler A. P.; Low K. C.; Sikes C. S. CaCO3 crystal-binding properties of peptides and their influence on crystal-growth. ACS Symp. Ser. 1991, 444, 72–84. 10.1021/bk-1991-0444.ch006. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
