Understanding the Solid-State Structure of Riboflavin through a Multitechnique Approach
- PMID: 39131447
- PMCID: PMC11311124
- DOI: 10.1021/acs.cgd.4c00480
Understanding the Solid-State Structure of Riboflavin through a Multitechnique Approach
Abstract
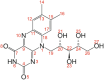
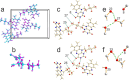
Crystalline riboflavin (vitamin B2) performs an important biological role as an optically functional material in the tapetum lucidum of certain animals, notably lemurs and cats. The tapetum lucidum is a reflecting layer behind the retina, which serves to enhance photon capture and vision in low-light settings. Motivated by the aim of rationalizing its biological role, and given that the structure of biogenic solid-state riboflavin remains unknown, we have used a range of experimental and computational techniques to determine the solid-state structure of synthetic riboflavin. Our multitechnique approach included microcrystal XRD, powder XRD, three-dimensional electron diffraction (3D-ED), high-resolution solid-state 13C NMR spectroscopy, and dispersion-augmented density functional theory (DFT-D) calculations. Although an independent report of the crystal structure of riboflavin was published recently, our structural investigations reported herein provide a different interpretation of the intermolecular hydrogen-bonding arrangement in this material, supported by all the experimental and computational approaches utilized in our study. We also discuss, more generally, potential pitfalls that may arise in applying DFT-D geometry optimization as a bridging step between structure solution and Rietveld refinement in the structure determination of hydrogen-bonded materials from powder XRD data. Finally, we report experimental and computational values for the refractive index of riboflavin, with implications for its optical function.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
A structure determination protocol based on combined analysis of 3D-ED data, powder XRD data, solid-state NMR data and DFT-D calculations reveals the structure of a new polymorph of l-tyrosine.Chem Sci. 2022 Mar 30;13(18):5277-5288. doi: 10.1039/d1sc06467c. eCollection 2022 May 11. Chem Sci. 2022. PMID: 35655549 Free PMC article.
-
Solid-State Structural Properties of Alloxazine Determined from Powder XRD Data in Conjunction with DFT-D Calculations and Solid-State NMR Spectroscopy: Unraveling the Tautomeric Identity and Pathways for Tautomeric Interconversion.Cryst Growth Des. 2022 Jan 5;22(1):524-534. doi: 10.1021/acs.cgd.1c01114. Epub 2021 Nov 22. Cryst Growth Des. 2022. PMID: 35024003 Free PMC article.
-
Ambiguous structure determination from powder data: four different structural models of 4,11-di-fluoro-quinacridone with similar X-ray powder patterns, fit to the PDF, SSNMR and DFT-D.IUCrJ. 2022 May 14;9(Pt 4):406-424. doi: 10.1107/S2052252522004237. eCollection 2022 Jul 1. IUCrJ. 2022. PMID: 35844476 Free PMC article.
-
Determination of a complex crystal structure in the absence of single crystals: analysis of powder X-ray diffraction data, guided by solid-state NMR and periodic DFT calculations, reveals a new 2'-deoxyguanosine structural motif.Chem Sci. 2017 May 1;8(5):3971-3979. doi: 10.1039/c7sc00587c. Epub 2017 Mar 16. Chem Sci. 2017. PMID: 28553539 Free PMC article.
-
Hydrogen Atomic Positions of O-H···O Hydrogen Bonds in Solution and in the Solid State: The Synergy of Quantum Chemical Calculations with ¹H-NMR Chemical Shifts and X-ray Diffraction Methods.Molecules. 2017 Mar 7;22(3):415. doi: 10.3390/molecules22030415. Molecules. 2017. PMID: 28272366 Free PMC article. Review.
References
-
- Gur D.; Palmer B. A.; Weiner S.; Addadi L. Light Manipulation by Guanine Crystals in Organisms: Biogenic Scatterers, Mirrors, Multilayer Reflectors and Photonic Crystals. Adv. Mater. 2017, 27, 1603514.10.1002/adfm.201603514. - DOI
-
- Wagner A.; Wen Q.; Pinsk N.; Palmer B. A. Functional Molecular Crystals in Biology. Isr. J. Chem. 2021, 61, 668–678. 10.1002/ijch.202100069. - DOI
-
- Palmer B. A.; Hirsch A.; Brumfeld V.; Aflalo E. D.; Pinkas I.; Sagi A.; Rosenne S.; Oron D.; Leiserowitz L.; Kronik L.; Weiner S.; Addadi L. Optically Functional Isoxanthopterin Crystals in the Mirrored Eyes of Decapod Crustaceans. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 2299–2304. 10.1073/pnas.1722531115. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
