Left Atrial Appendage Occlusion: Current Stroke Prevention Strategies and a Shift Toward Data-Driven, Patient-Specific Approaches
- PMID: 39131471
- PMCID: PMC11308563
- DOI: 10.1016/j.jscai.2022.100405
Left Atrial Appendage Occlusion: Current Stroke Prevention Strategies and a Shift Toward Data-Driven, Patient-Specific Approaches
Abstract
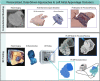
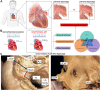
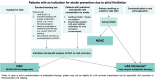
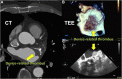
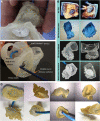
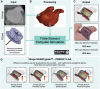
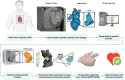
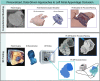
The left atrial appendage (LAA) is a complex structure with unknown physiologic function protruding from the main body of the left atrium. In patients with atrial fibrillation, the left atrium does not contract effectively. Insufficient atrial and LAA contractility predisposes the LAA morphology to hemostasis and thrombus formation, leading to an increased risk of cardioembolic events. Oral anticoagulation therapies are the mainstay of stroke prevention options for patients; however, not all patients are candidates for long-term oral anticoagulation. Percutaneous occlusion devices are an attractive alternative to long-term anticoagulation therapy, although they are not without limitations, such as peri-implant leakage and device-related thrombosis. Although efforts have been made to reduce these risks, significant interpatient heterogeneity inevitably yields some degree of device-anatomy mismatch that is difficult to resolve using current devices and can ultimately lead to insufficient occlusion and poor patient outcomes. In this state-of-the-art review, we evaluated the anatomy of the LAA as well as the current pathophysiologic understanding and stroke prevention strategies used in the management of the risk of stroke associated with atrial fibrillation. We highlighted recent advances in computed tomography imaging, preprocedural planning, computational modeling, and novel additive manufacturing techniques, which represent the tools needed for a paradigm shift toward patient-centric LAA occlusion. Together, we envisage that these techniques will facilitate a pipeline from the imaging of patient anatomy to patient-specific computational and bench-top models that enable customized, data-driven approaches for LAA occlusion that are engineered specifically to meet each patient's unique needs.
Keywords: computed tomography; imaging; left atrial appendage; left atrial appendage occlusion; stroke.
© 2022 The Authors.
Conflict of interest statement
Dr Wang is a consultant or advisory to Edwards Lifesciences Corporation, Boston Scientific Corp, Abbott, and Neochord Inc and receives funding grants from Boston Scientific Corp. Dr O’Neill is a consultant or advisory to Edwards Lifesciences Corporation and Abbott. Prof. Roche is a consultant to Holistick Medical and Surmodics and is on the board of directors for Affluent Medical and the scientific advisory board for Helios Cardiovascular. Ms Mendez and Mr Kennedy reported no financial interests.
Figures


References
-
- Tabata T., Oki T., Yamada H., et al. Role of left atrial appendage in left atrial reservoir function as evaluated by left atrial appendage clamping during cardiac surgery. Am J Cardiol. 1998;81(3):327–332. - PubMed
-
- Beigel R., Wunderlich N.C., Ho S.Y., Arsanjani R., Siegel R.J. The left atrial appendage: anatomy, function, and noninvasive evaluation. J Am Coll Cardiol Imaging. 2014;7(12):1251–1265. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

